Attacking FLT3 Mutations Yields First Targeted Therapy in AML
Although FLT3 mutations are well established as a prognostic marker in patients with AML, efforts to target the aberration therapeutically were underway for more than 15 years before yielding success.
Elias Jabbour, MD

Elias Jabbour, MD
Although FLT3 mutations are well established as a prognostic marker in patients with acute myeloid leukemia (AML), efforts to target the aberration therapeutically were underway for more than 15 years before yielding success. That happened in April, when the FDA approved midostaurin (Rydapt), the first new therapy for AML in about 40 years.
Midostaurin is approved for the treatment of adult patients with newly diagnosed FLT3-positive AML in combination with standard cytarabine and daunorubicin induction and cytarabine consolidation. The drug has also been approved for patients with advanced systemic mastocytosis (SM), including aggressive systemic mastocytosis (ASM), SM with associated hematological neoplasm (SM-AHN), and mast cell leukemia.
AML is a genetically complex disease that researchers are still seeking to elucidate. Researchers believe that AML is driven by somatic alterations in a “2 hit” process: a proliferative mutation in a class I gene such as FLT3 and an aberration in a class II gene that prevents cells from maturing.1
FLT3 mutations are among the epigenetic drivers of AML in normal karyotypes (Figure).2 Investigators have found somatic mutations in FLT3 genes in several spots, notably in 27% to 34% of samples in the internal tandem duplication (ITD) domain in various studies.1 The presence of an FLT3-ITD mutation confers a poor risk for patients with AML, with worse outcomes in diseasefree and overall survival.3
In recently updated joint guidelines, the College of American Pathologists and the American Society of Hematology strongly recommend testing all pediatric and adult patients with suspected or confirmed AML for FLT3-ITD mutations.3 Midostaurin was approved along with a companion diagnostic, the LeukoStrat CDx FLT3 Mutation Assay, to test for FLT3 mutations in DNA extracted from mononuclear cells obtained from peripheral blood or bone marrow aspirates.
Although it was approved for patients with FLT3 mutations, midostaurin is a small molecule that inhibits multiple receptor tyrosine kinases; in vitro biochemical and cellular assays also have suggested that it can inhibit wild-type FLT3 activity.4 In reference to aberrant FLT3 activity, the drug inhibits FLT3 receptor signaling and cell proliferation, and induces apoptosis in leukemic cells expressing ITD and tyrosine kinase domain mutant FLT3 receptors.4
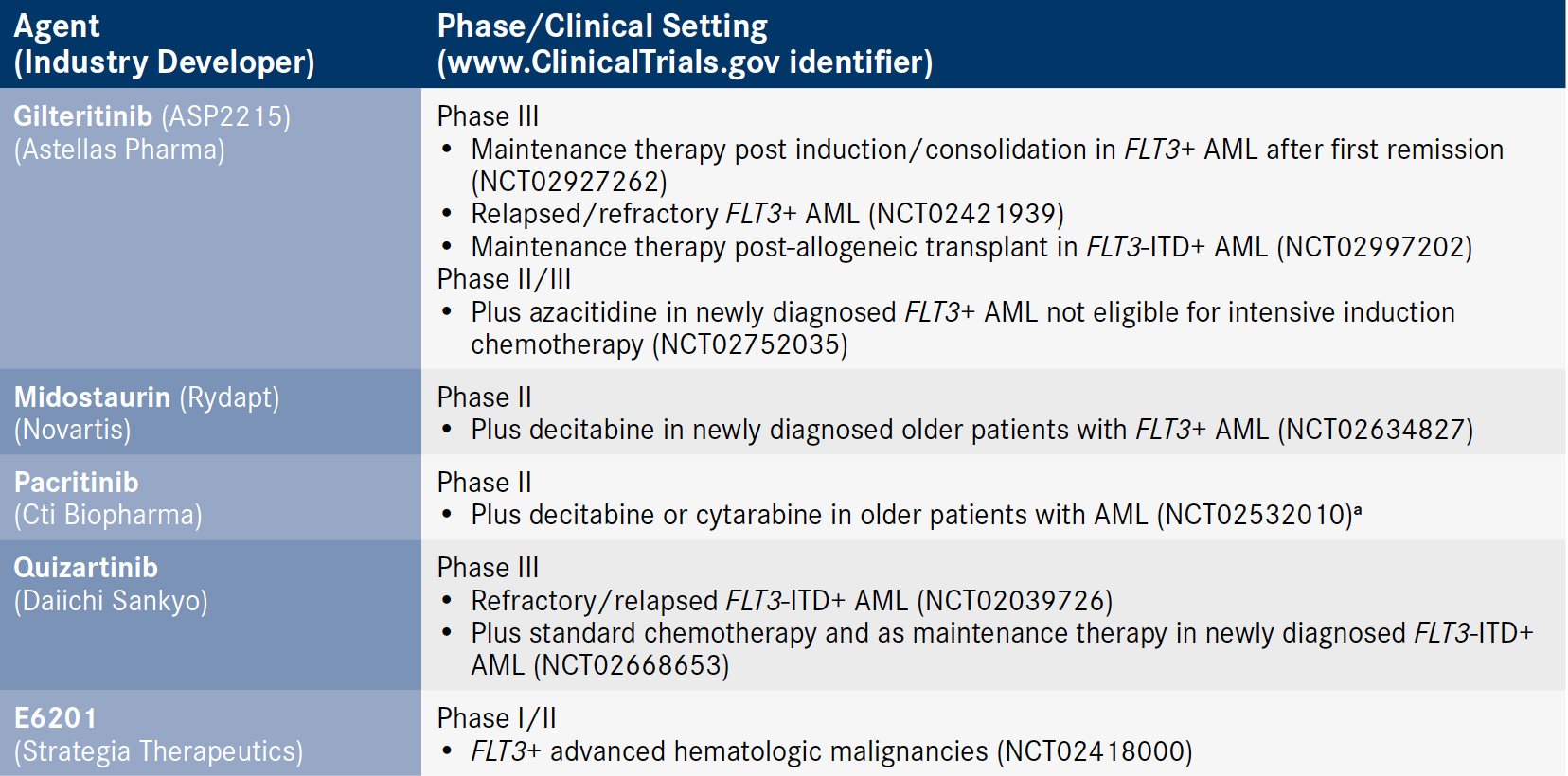
The midostaurin approval has generated much excitement in the field. Several other FLT3 inhibitors have entered the later-stages of clinical development (Table) and the prospects for more targeted agents are building.
Table. Selected Later-Stage Clinical Trials of FLT3 Inhibitors in AML
“Use of next-generation sequencing technology has generated a plethora of novel insights into the genetics of AML, providing important information about dysregulated signaling involved in leukemic transformation and leading to new therapeutic targets,” said Elias Jabbour, MD, an associate professor at The University of Texas MD Anderson Cancer Center in Houston, during a recent OncLive® Peer Exchange® program.5 “After a dearth of new therapies available for acute myeloid leukemia over the last few decades, we are transitioning into a new era with several promising strategies in latestage development.”
aStudy is ongoing but not recruiting participants. AML indicates acute myeloid leukemia; FLT, FMS-like tyrosine kinase 3; ITD, internal tandem duplication.
Pivotal Midostaurin Findings
The midostaurin approval is based on the phase III RATIFY trial in AML and 2 single-arm, open-label studies of patients with SM. In RATIFY, the addition of midostaurin to standard chemotherapy reduced the risk of death by 23% compared with chemotherapy alone in patients with FLT3-mutant AML. After censoring for patients who received stem cell transplants, the overall survival (OS) benefit with midostaurin remained steady at 25%.
In the phase II trial considered for the SM approvals, among patients receiving 6 cycles of midostaurin, the rates of confirmed complete remission (CR) plus incomplete remission (ICR) by modified Valent criteria were 38% for ASM and 16% for SM-AHN. One patient with mast cell leukemia had a CR.
In the phase III RATIFY trial, also known as CALGB 10603, 717 patients with newly diagnosed FLT3-mutant AML were randomized to standard induction and consolidation chemotherapy plus midostaurin (n = 360) or placebo (n = 357). Hydroxyurea was allowed for up to 5 days prior to beginning therapy, while FLT3 test results were obtained.
During induction therapy, daunorubicin was given at 60 mg/m2 on days 1 to 3 with cytarabine at 200 mg/m2 on days 1 to 7. Oral midostaurin was administered at 50 mg twice daily on days 8 to 22. If patients achieved a complete remission, consolidation therapy was given with cytarabine at 3 g/m2 for 3 hours every 12 hours on days 1, 3, and 5 plus either placebo or midostaurin. After 4 cycles of consolidation, maintenance therapy with either midostaurin or placebo was administered for up to 1 year.
The 2 treatment arms were well balanced for age (median, 48 years), race, FLT3 subtype, and baseline complete blood counts. There were more males in the midostaurin arm versus placebo (48.2% vs 40.6%). The primary endpoint of the study was OS, with secondary outcome measures such as event-free survival (EFS) and safety.
In uncensored data, median OS was 74.7 months with midostaurin versus 25.6 months with chemotherapy alone (HR, 0.77; 95% CI, 0.63-0.95; P = .016). The 5-year OS rate for patients in the midostaurin arm was 50.9% versus 43.9% with placebo. Median EFS with midostaurin was 8.2 months versus 3.0 months with placebo (HR, 0.78; 95% CI, 0.66-0.93; P = .004). The 5-year EFS with midostaurin was 27.5% versus 19.3% with placebo.
Median OS seen in the midostaurin arm was well beyond investigator expectations of 20.9 months.A possible explanation for this could be the rates of stem cell transplantation or incomplete data. The confidence intervals for OS were not fully attained for the midostaurin arm (95% CI, 31.7-not attained).
Overall, 57% of patients received an allogeneic stem cell transplant at some time during the trial, more commonly in the midostaurin arm versus placebo (58% vs 54%). Median time to transplant was 5.0 months with midostaurin and 4.5 months with placebo. Twenty-five percent of transplants occurred during the first complete remission. Overall, 59% of patients in the midostaurin arm and 54% in the placebo group experienced a complete remission (P = .18).
Median OS data were not obtained in the censored population. Overall, the 4-year censored OS rate with midostaurin was 63.8% versus 55.7% for placebo (HR, 0.75; P = .04). In those censored for transplant, median EFS with midostaurin was 8.2 months versus 3.0 months with placebo (HR, 0.84; P = .025).
Grade ≥3 AEs were similar between the midostaurin and placebo arms. Overall, 37 grade 5 AEs occurred in the study, which were similar between the 2 arms, at 5.3% with midostaurin versus 5.0% with placebo. A statistically significant difference was not observed for treatment-related grade 5 AEs (P = .82).
References
- Seegmiller, A., M. Jagasia, S. Wheeler, C. Vnencak-Jones. 2016. Molecular Profiling of Acute Myeloid Leukemia. My Cancer Genome website. www.mycancergenome.org/content/disease/acute-myeloid-leukemia. Updated January 26, 2016. Accessed May 31, 2017.
- Mehdipour P, Santoro F, Minucci S. Epigenetic alterations in acute myeloid leukemias. FEBS Journal. 2015;282(9):1786—1800. doi: 10.1111/febs.13142.
- Arber DA, Borowitz MJ, Cessna M, et al. Initial diagnostic workup of acute leukemia: guideline from the College of American Pathologists and the American Society of Hematology [published online February 22, 2017]. Arch Pathol Lab Med. doi: 10.5858/arpa.2016-0504-CP.
- Rydapt [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017.
- OncLive Peer Exchange. Moving toward precision medicine in adult acute myeloid leukemia. OncLive.com website. onclive.com/link/1192. Published January 10, 2017. Accessed May 31, 2017.




