Who Is at the Controls of 340B?
The 340B Drug Pricing Program, designed to provide support for out-patient drug purchases, severely lacks federal oversight, said BRG Healthcare, the business advisory group, in a report sponsored by the Alliance for Integrity and Reform of 340B (AIR340B).
The 340B Drug Pricing Program, designed to provide support for outpatient drug purchases, severely lacks federal oversight, said BRG Healthcare, the business advisory group, in a report sponsored by the Alliance for Integrity and Reform of 340B (AIR340B). BRG increased its growth estimates for 340B and said the program would exceed $23 billion in total sales by 2021 (Figure 1). That amount is higher than the total Medicare Part B drug reimbursement in 2014, and the comparison is significant, BRG said, because Medicare Part B currently has significantly more administrative assistance from the Health Resources & Service Administration (HRSA) than 340B receives.
AIR340B is a coalition of patient advocacy groups, clinical care providers, and pharmaceutical companies that contend the 340B program has been stretched far beyond its original mandate to provide discounted drugs for uninsured indigent patients. The group includes the Community Oncology Alliance, which has stated that promoting 340B reform is among its goals for 2017. Drug industry observers have also said political inclination in Washington, DC, to change 340B is weak. One of the groups strongly in support of 340B is the 340B Coalition, which contends that 340B is an important safety net for patients that provides participating hospitals with an important source of funding for charity programs.
340B allows qualifying providers to obtain drugs from manufacturers at deep discounts. It is not necessary for those providers to lower what they charge for that medicine, and the margins on sales make it easier for hospitals and other institutions to provide comprehensive care for those who need it. Critics allege that those 340B savings are being pocketed or misused by unscrupulous providers and that providers who do not qualify for 340B participation are placed at a competitive disadvantage. The potential for financial gain and the lack of regulatory control are seen as major reasons for the extraordinary growth of the program.
BRG said 340B is overseen by an agency that has just 22 part-time auditors who, by 2021, will each be responsible for watching over 4000 entities and more than $1 billion in 340B drug sales. “The 340B program is currently administered by HRSA’s Office of Pharmacy Affairs, which operates on an annual budget of approximately $10 million. This pales in comparison to the $733 million in federal administrative budget for Medicare and Medicaid and highlights the challenges HRSA faces in effectively regulating the 340B program,” BRG said. Compliance issues—for example, drug discounts being applied in cases where charitable assistance is not necessary—have been identified as rife by government investigations.
The 340B program requires drug manufacturers to provide discounts as deep as 50% on drugs prescribed by participating providers. However, the program is criticized because, in many cases, these discounts are not passed along to patients. Providers sometimes do not apply the difference between the discount and the regular drug price toward charitable care. Critics say the program’s enormous profit potential has contributed to huge expansion of hospital networks. Since the beginning of 2015, over 390 hospitals have enrolled in the program for the first time, BRG said.
The 340B program is also blamed for acquisitions by hospitals of private practices and exacerbation of business difficulties for independent practices that do not have access to the program.
BRG said that “340B hospitals have grown from accounting for 13% of Medicare Part B drug reimbursement in 2010 to 25% by 2015. Part of this growth is attributable to new hospital enrollments, but over half of the growth is due to expanded drug utilization at existing 340B hospitals. The result is a shift in site of care to the higher-cost outpatient setting that is driven, at least in part, by the sizeable margins realized by hospitals on drugs purchased through the 340B program.” For example, Part B drug payments to hospitals that were enrolled in 340B prior to 2010 totalled $1.8 billion in 2010 and $3.9 billion in 2015, the report said. Meanwhile, among hospitals that enrolled in 340B in 2010 or later, Medicare Part B payments rose from $0.1 billion in 2010 to $1.9 billion in 2015.
New guidance from HRSA is anticipated on how 340B should be implemented, and this could slow the growth of the program or even reduce its size, the report said. A public comment period designed to allow stakeholders to weigh in on potential changes to the program ended in late 2016. The size of 340B also could be reduced if more biosimilar drugs, which are cheaper substitutes for brand name drugs, are launched in the marketplace, the report said. BRG also suggested that the now-defunct Medicare Part B Drug Payment Model may have had an impact on 340B sales. The proposed CMS program, which would have changed the drug payment formula for physicians, was abandoned in December by the Obama administration as the Republican administration prepared to take office. Weak political support for the drug pricing model was seen as the cause of the abandonment.
The new report from AIR340B states that BRG researchers had to upwardly revise their earlier estimates of 340B sales growth. Their 2014 forecast showed sales reaching $16 billion by 2019; that number is now obsolete. Based on current growth, sales could reach $20.8 billion in 2019, up from an anticipated $16.1 billion in 2016, BRG said. Among the components of the revised growth estimate are sales attributable to hospital acquisitions of private practices, new physician-hospital affiliations, and expanded hospital referral networks. As a group, these constituted the biggest change in the forecast and are expected to total $1 billion for 2016 and reach $5 billion in 2021, thereby eclipsing the growth of contract pharmacy sales, another rapidly growing element of 340B sales.
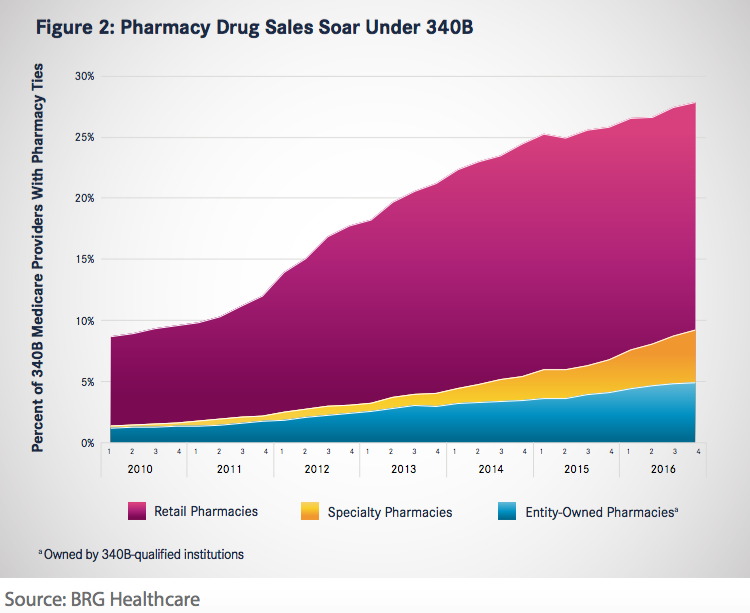
Contract pharmacy sales growth through 340B is expected to measure $1 billion in 2016 and $4.6 billion in 2021. Hospital pharmacy arrangements have soared as a result of 340B impetus (Figure 2). That growth can be traced back to 2010 guidance from HRSA that allowed covered 340B entities to contract with an unlimited number of third-party pharmacies to dispense 340B drugs. By 2016, over 68% of 340B-participating hospitals had at least 1 contract pharmacy arrangement, up from 13% in 2010. “Although there is a natural limit to overall participation rates, our research indicates emerging growth trends in specialty pharmacies and covered entity ownership of contract pharmacies. Therefore, we expect contract pharmacies to continue to drive incremental 340B sales for at least the next 5 years,” the report said.
In further conclusions, BRG said there may be unanticipated ripple effects from the dramatic growth of 340B drug discounting. The program may already be causing manufacturers to charge higher overall drug prices, thereby driving up the costs of healthcare, BRG stated.
Vandervelde A, Blalock E. 340B Program sales forecast: 2016 - 2021. AIR340B website. http://340breform.org/userfiles/December%202016%20 BRG%20Growth%20Study.pdf. Published 2016. Accessed January 9, 2017.




