Nivolumab Combos Explored in First-Line NSCLC Trial
Although nivolumab has demonstrated a clear survival advantage compared with chemotherapy in patients with progressive non–small cell lung cancer who express PD-L1 in their tumor cells, the same cannot be said for those who are PD-L1–negative.
Leora Horn, MD, MSc

Leora Horn, MD, MSc
Although nivolumab (Opdivo) has demonstrated a clear survival advantage compared with chemotherapy in patients with progressive non—small cell lung cancer (NSCLC) who express PD-L1 in their tumor cells, the same cannot be said for those who are PD-L1–negative.1
As a result, researchers are seeking to elicit antitumor activity in a broader range of patients, notably through a multiarm trial evaluating the PD-1 inhibitor along with combinatorial approaches.
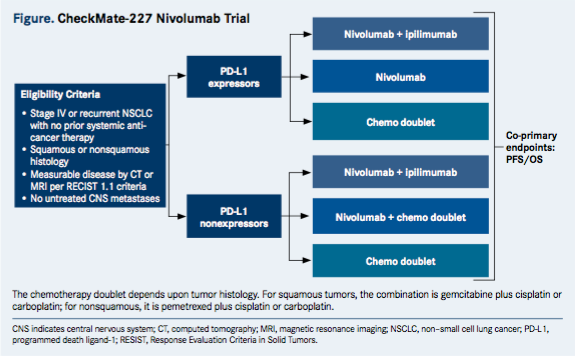
CheckMate-227 (NCT02477826) is a phase III, open-label, randomized trial for patients with chemotherapy-naïve stage IV or recurrent NSCLC. The trial will enroll patients into separate groups according to PD-L1 expression status (≥1% or <1%).
Participants who are PD-L1—positive are randomized to receive nivolumab monotherapy, nivolumab plus the CTLA-4 inhibitor ipilimumab (Yervoy), or platinum-doublet chemotherapy. Those who are PD-L1–negative receive either nivolumab plus ipilimumab, nivolumab plus chemotherapy, or a chemotherapy doublet. The choice of chemotherapy depends upon histology. (Figure).
Leora Horn, MD, MSc, said the trial is looking at whether combination therapy can improve upon nivolumab monotherapy for PD-L1—positive patients. “In the PD-L1–negative patients, we are asking whether there is any benefit to combination nivolumab and ipilimumab compared with chemotherapy in patients.”
Horn is clinical director of the Thoracic Oncology Program at Vanderbilt-Ingram Cancer Center. She is the principal investigator for the trial at Vanderbilt.
“Right now, chemotherapy is considered the standard of care for patients with advanced-stage NSCLC in the first-line setting,” said Suresh S. Ramalingam, MD. He is a principal investigator at Emory University, where he is assistant dean for Cancer Research at the School of Medicine, and deputy director of Winship Cancer Institute.
“Immune checkpoint inhibitors are approved in the second-line setting. The question then is, are there subsets of patients for whom immune checkpoint inhibitors can be used in the frontline setting?” said Ramalingam. “This is an ongoing study in the frontline setting of NSCLC, and it seeks to determine what the optimal therapy is based on PD-L1 expression status.”
To be eligible for CheckMate-227, patients must be 18 years old or older, have histologically confirmed stage IV or recurrent squamous or nonsquamous NSCLC with no prior systemic anticancer therapy, an ECOG performance status ≤1, and measurable disease by CT/MRI per response evaluation criteria in solid tumors. The trial is still open to enrollment. Nearly 2000 patients are expected to participate.
Mixed Results in Recent Trials
The CheckMate-227 trial is continuing amid disappointing results for nivolumab monotherapy in another phase III trial. In the CheckMate-026 trial, first-line therapy with nivolumab failed to meet the primary endpoint of superior PFS compared with chemotherapy.2
A spokeswoman for Bristol-Myers Squibb, which is developing nivolumab, said the CheckMate-227 trial will proceed as planned. She said CheckMate-026 answered important questions about anti-PD-1 monotherapy in a broad patient population, where there remains a high unmet need.
Meanwhile, the prospect of combining a PD-1 inhibitor and a CTLA-4 inhibitor is proving exciting.
In the CheckMate-012 study, upfront treatment with nivolumab plus ipilimumab demonstrated an objective response rate of 57% in patients with PD-L1—positive advanced NSCLC.3 Prior evidence in other indications, such as melanoma, suggests that the PD-1/CTLA-4 combination elicits activity in patients without PD-L1 expression, said Ramalingam.
“Essentially, you are trying to understand whether the combination approach of CTLA-4 and PD-1 targeting can be used in patients,” he said. “There are some lines of evidence that suggest that if you’re PD-1—negative and you’re given a CTLA-4 plus PD-1 inhibition, then the combination works well. Whereas, if we give a PD-1 inhibitor as monotherapy, the activity tends to be lower. The scientific question is, can we use the combination to overcome the potential effect of not having high PD-L1 expression within the tumor?”
Horn noted that many combination trials with immunotherapy agents are underway in NSCLC. “There is a whole area of active investigation in the first-line setting looking at checkpoint inhibitors in combination with other agents to see if we can increase that response rate from the traditional 25% that you see with chemotherapy—or the 10% to 20% that you see with an immune checkpoint inhibitor on its own—to increase those response rates and improve survival in patients with stage IV disease,” she said.
References
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non—small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi:10.1056/NEJMoa1507643.
- Socinski M, Creelan B, Horn L, et al. CheckMate 026: A phase 3 trial of nivolumab vs investigator’s choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage IV/recurrent programmed death ligand 1 (PD-L1)−positive NSCLC. Presented at: 2016 ESMO Congress; October 7-11, 2016; Copenhagen, Denmark. LBA7_PR.
- Hellmann MD, Gettinger SN, Goldman JW, et al. CheckMate 012: Safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. J Clin Oncol. 2016;34(suppl; abstr 3001).




