Myeloma Trial Explores Optimal Induction, Maintenance Regimens
Two combination regimens anchored by different proteasome inhibitors are being compared in a national clinical trial for patients with newly diagnosed symptomatic multiple myeloma that also seeks to evaluate the ideal duration of lenalidomide (Revlimid) maintenance therapy.
Shaji Kumar, MD
Two combination regimens anchored by different proteasome inhibitors are being compared in a national clinical trial for patients with newly diagnosed symptomatic multiple myeloma (MM) that also seeks to evaluate the ideal duration of lenalidomide (Revlimid) maintenance therapy.
The phase III ENDURANCE trial (NCT01863550), which is currently enrolling and has accrued nearly 600 patients, will randomize participants to receive lenalidomide and dexamethasone with either bortezomib (Velcade) or carfilzomib (Kyprolis) for 9 months, followed by lenalidomide maintenance therapy administered either indefinitely or for 2 years (Figure).
The primary endpoint of the trial is overall survival (OS) for the maintenance analysis. Secondary endpoints include progression-free survival (PFS) for the maintenance phase and PFS for the induction stage.
“At this point, we don’t know how long [lenalidomide maintenance] needs to be given, so this time we’re going to look at whether 2 years is sufficient or if we need to give it longer. This will definitely have clinical value because if we can get away with limited duration of treatment, it helps reduce costs and also helps reduce toxicity,” said Shaji Kumar, MD, a professor of medicine at Mayo Clinic in Rochester, Minnesota, who is the national principal investigator for the trial.
The study is open to patients with newly diagnosed, stage I-III MM who have not received prior treatment for their disease. The trial will enroll patients categorized as standard risk, with no evidence of high-risk chromosomal abnormalities, which are defined as the translocations t(4;14), t(14;16) and t(14;20) or deletion 17p. Patients cannot have received more than 1 cycle of prior chemotherapy and no more than 160 mg of prior dexamethasone for treatment of symptomatic myeloma. They also should not have been treated with lenalidomide, bortezomib, or carfilzomib.
Figure
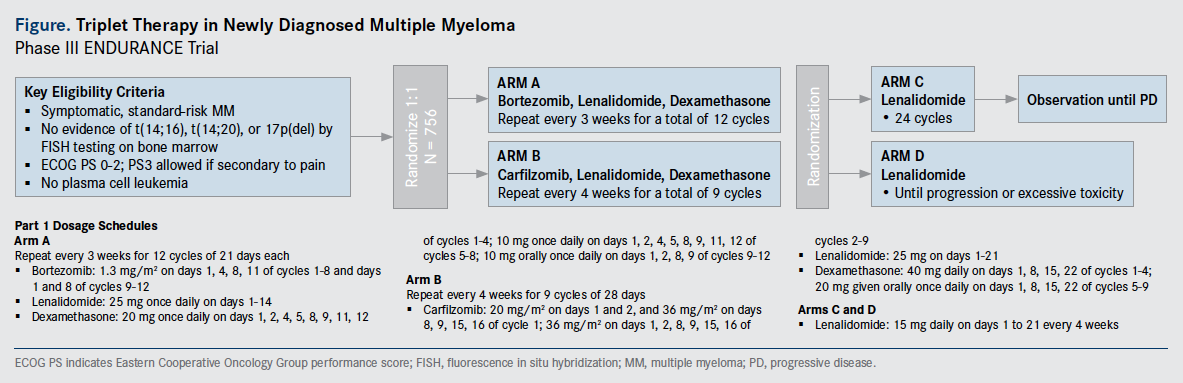
“Patients who are eligible can undergo a stem cell collecton for transplantation at the time of disease relapse, and then can continue on the trial as planned,” explained Kumar. Only patients who are at standard-risk are eligible for this study because there is a parallel trial investigating high-risk patients.
In comparing induction strategies, the study is evaluating triplet regiments containing bortezomib and carfilzomib, the competing proteasome inhibitors. Although both agents affect the same 26S proteasome target, bortezomib is a reversible inhibitor, whereas carfilzomib is not. Due to this difference, investigators are interested in seeing whether carfilzomib can more effectively kill tumor cells or block them from dividing, making it a potentially better treatment for patients with MM.
The combination of bortezomib with lenalidomide and dexamethasone has become a standard regimen as primary therapy for transplant and nontransplant candidates, and carries a category 1 recommendation in the National Comprehensive Cancer Network guidelines.1 Carfilzomib, a second-generation proteasome inhibitor that the FDA has approved in the relapsed and refractory settings, has demonstrated high PFS rates in newly diagnosed patients in phase I-II clinical trials, resulting in a category 2A option as a primary treatment for transplant-eligible patients.1
The most common adverse events (AEs) associated with bortezomib have been decreases in blood counts, particularly platelet counts; peripheral neuropathy; and increased risk of infections. For carfilzomib, common AEs include drops in blood counts, increased risk of infections, and fatigue. There also was a higher incidence of cardiac failure with the combination of carfilzomib and dexamethasone versus bortezomib and dexamethasone in a previous clinical trial.2 In the head-to-head portion of the ENDURANCE trial, investigators will be closely monitoring patients for these AEs, Kumar said.
The ENDURANCE trial is being sponsored by the ECOG-ACRIN Cancer Research Group, with collaboration from the National Cancer Institute. Bortezomib is developed by Takeda Oncology, and carfilzomib is developed by Amgen Inc.
References
- NCCN clinical practice guidelines in oncology: multiple myeloma version 3.2017. National Comprehensive Cancer network website. nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Updated November 28, 2016. Accessed July 10, 2017.
- Kyprolis [prescribing information]. Thousand Oaks, CA: Onyx Pharmaceuticals, Inc; 2017.




