Article
COVID-19: The Unexpected Sequelae
Author(s):
History will report that the COVID-19 pandemic in Brooklyn, New York peaked on or about April 12, 2020; however, due to the protracted hospitalization experienced by severe COVID-19–positive patients, the heavy in-patient burden continues today.
Patrick Ivan Borgen, MD, Chairman, Department of Surgery Maimonides Medical Center Brooklyn, New York

On May 7, 2020, Maimonides Medical Center discharged our 1000th successfully treated COVID-19–positive inpatient. History will report that the COVID-19 pandemic in Brooklyn, New York peaked on or about April 12, 2020; however, due to the protracted hospitalization experienced by severe COVID-19–positive patients, the heavy in-patient burden continues today.
One month after the peak incidence, our hospital still is managing over 100 patients on ventilators for COVID-19–associated pulmonary collapse. Maimonides Medical Center, located in South Brooklyn, was at the very epicenter of the US COVID-19 crisis. Three of our neighborhood zip codes ranked in the top 7 in the state of New York—the region hit the hardest by the virus. Greater New York comprises only 3% of the US population, but shouldered 35% of all COVID-19–related deaths to date.1 The actual percentage is almost certainly higher than that number as thousands of citizens died at home, the majority with COVID-19–related illnesses. The New York Fire Department reported that the number of bodies retrieved from private residences was 8 times the normal number, and many were due to an unexpected sequelae of the viral infection—a severely hypercoagulable state.
Many of these victims, who never actually became patients, died of complications of the remarkable hypercoagulable state that is associated with this novel coronavirus. We witnessed the entire panorama of clotting sequelae including ischemic limbs, stroke, myocardial infarction, visceral ischemia, and pulmonary embolism. Multiple reports of central line clotting during the insertion procedure surfaced, and clinical teams cited more and more examples of hypercoagulation. Vascular surgical interventions soared, as did the need for limb amputations.
The abnormal blood clotting diathesis has been reported from a number of countries, including the Netherlands and France. These reports suggest that clots appear in 20% to 30% of critically ill COVID-19 patients despite attempts at conventional anti-coagulation.2,3 One of the earliest observations that lead to the discovery of this blood clotting issue was the reports that young people were dying of strokes caused by the blockages in the brain. We have found that many patients have drastically elevated levels of a protein fragment called D-dimer, which is generated when a blood clot dissolves. We are investigating whether high levels of D-dimer might serve as an early warning sign for coagulation related mortality in hospitalized patients infected with coronavirus.
Researchers at Weill Cornell Medicine in New York City have observed miniature clots in the body’s smallest vessels. Jeffrey Laurence, MD, a hematologist oncologist and his colleagues examined lung and skin samples from three people infected with COVID-19 and found that the capillaries were clogged with clots.4
Seemingly paradoxically, regional hospitals in New York reported a striking decline in common conditions, such as ST-segment elevation myocardial infarctions (STEMIs) and stroke. Lay press articles even suggested that COVID-19 was somehow “protective” against stroke and heart attack. We now know that patients were dying at home, rather than seeking care during the pandemic.
A survey of 7 metropolitan New York hospitals revealed a striking decline in Type A aortic dissections, presenting for emergent surgical intervention from a running 6-week average of 13, down to 3 over the same time period.5 Patients arriving at the hospital with acute myocardial infarction were often found to demonstrate widespread clotting that was not amenable to angiographic intervention, leading our center and others to use older strategies including tissue plasminogen activator, which is a protein involved in the breakdown of blood clots. Its mechanism of action includes catalyzing conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Most commonly, TPA has been used to treat patients with embolic or thrombotic stroke. The use of this protein is contraindicated in hemorrhagic stroke. TPA was used historically for myocardial infarction but was rapidly supplanted by targeted angiography with balloon angioplasty and stent placement.
Why this clotting occurs is still a mystery. One possibility is that the COVID-19 virus is directly attacking the endothelial cells that line our blood vessels. Endothelial cells express the same angiotensin converting enzyme (ACE-2) receptor that the virus uses to enter lung cells and these endothelial cells can become directly infected.
The virus’ effects on the immune system could also be responsible for this clotting. The viral “pneumonia” that is often the cause of death in patients with severe COVID-19 is actually pulmonary damage, which is caused by a massive immune response that has been called cytokine storm. This inflammatory response appears to activate the complement system, a natural defense mechanism that can trigger clotting. Inflammation, complement activation, and clotting are almost certainly related in patients with COVID-19.
These findings lead us to significantly revise our algorithm for anticoagulation in COVID-19–positive or suspected patients. (Figure)
Initially we employed 2 criteria for consideration of anticoagulation:
- Patients on high flow oxygen or mechanical ventilatory support who develop sudden clinical and laboratory findings highly consistent with pulmonary embolism, especially when chest x-ray and/or markers of inflammation are stable or improving. Pulmonary damage from cytokine storm is common late in COVID-19 and may also be associated with pulmonary microcapillary thrombosis.
- Patients with respiratory failure, particularly when D-dimer and/or fibrinogen levels are very high, in whom pulmonary embolism or microvascular thrombosis is highly suspected and other causes are not identified (such as acute respiratory distress syndrome, fluid overload).
Figure. COVID-19 Anticoagulation Algorithum
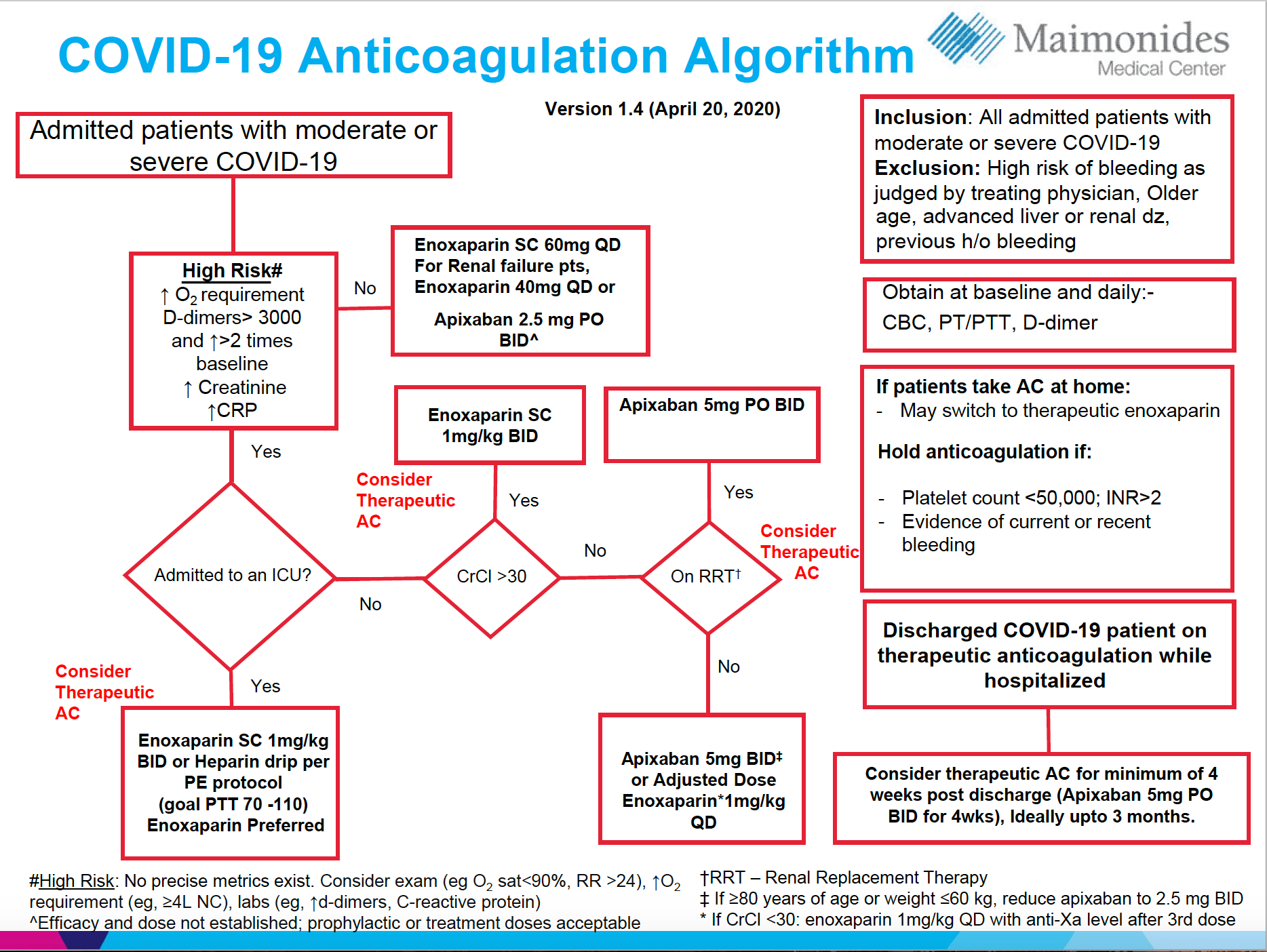
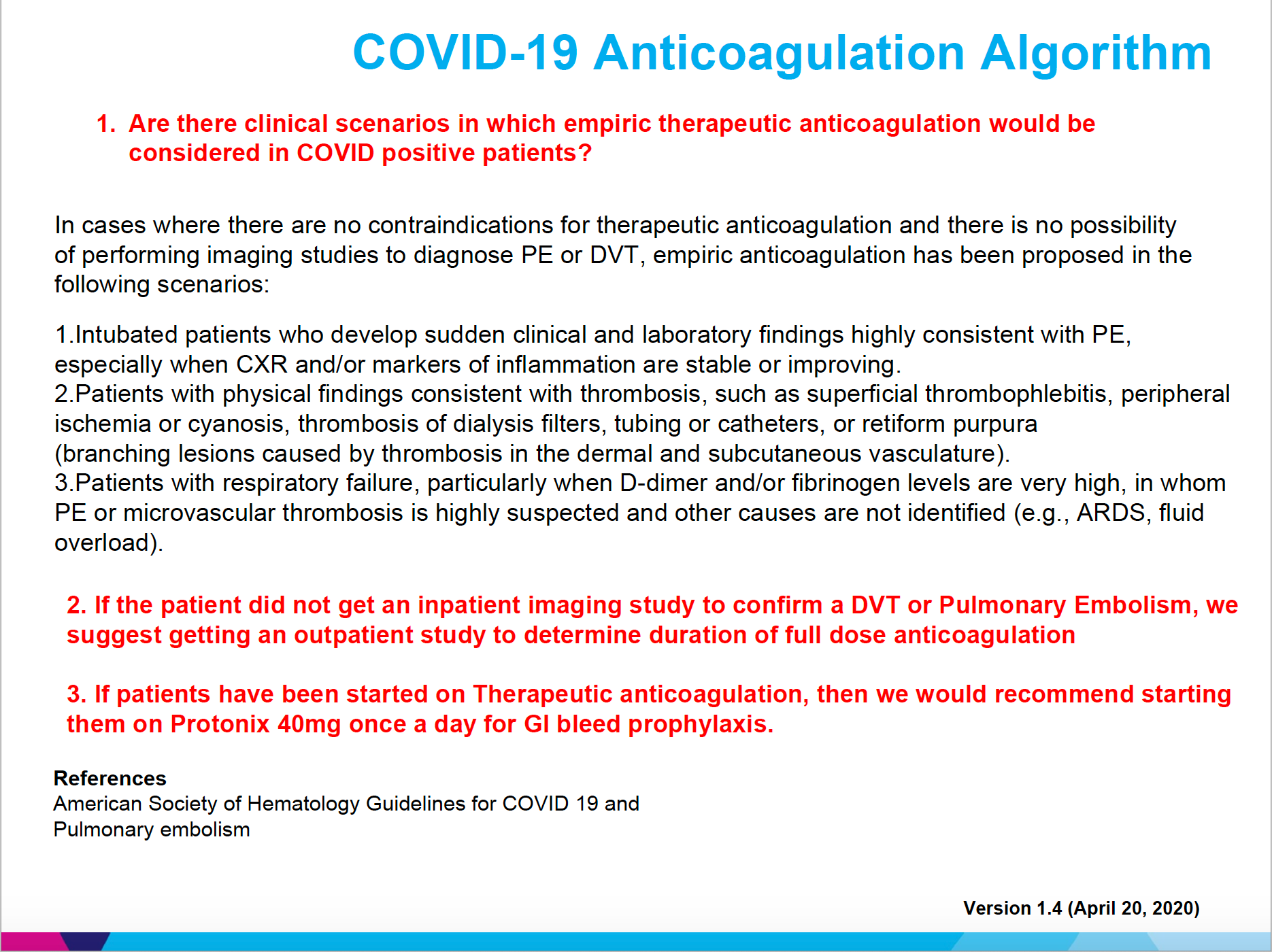
Our clinical experience led us to surmise that the coagulopathy was often occurring earlier in the disease process and we now anticoagulants considerably sooner if there is no other relative contraindication. We have also begun discharging patients on 3-month anti-coagulation prophylaxis.
Pandemics historically have functioned to refract the light of who we really are as human beings. This prism shines light on our own mortality, our relationship with the environment and the symbiotic relationship that we have with each and every other human on the planet. We have learned that we are all in this together. We have had to learn about this vicious virus on the fly, including most surprisingly, the unexpected hypercoagulable state that has taken so many lives.
There is still no proven therapy for COVID-19, and much of the available ongoing research has been limited by lack of scientific discipline, which has been driven by the extraordinary need to intervene in a crisis. It is possible that the biggest thing we learned was about the nature of our response and the very human flaws—driven by our humanity—and the super-human heroics that were revealed by this pandemic. There is still much to learn about COVID-19 and how we can better handle the next pandemic.
Editor’s Note: This column is the third in a series by Patrick I. Borgen, MD, on the COVID-19 pandemic and how it is impacting the healthcare system.
References
- COVID-19: Data: New York City. https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Published 2020.
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online ahead of print April 10, 2020]. Thromb Res. doi:10.1016/j.thromres.2020.04.013
- Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence [published online ahead of print April 24, 2020]. Circul. doi:10.1161/CIRCULATIONAHA.120.047430
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases [published online ahead of print April 15, 2020]. Transl Res. doi:10.1016/j.trsl.2020.04.00









