Publication
Article
Exploring Tumor Treating Fields Therapy
THIS ARTICLE IS SPONSORED BY NOVOCURE®. This article includes expert insights from Moshe Giladi, PhD; and Narasimha Kumar Karanam, PhD, BCMAS. Giladi, Novocure’s Chief Science Officer, oversees Novocure’s preclinical research organization and manages external research partnerships and Novocure’s biostatistics and data management teams. Karanam is Novocure’s Medical Science Liaison and has researched the mechanisms of action underlying tumor treating fields therapy.
Despite advances in prevention and treatment, in the United States an estimated 1670 people will die each day from cancer.3 Tumor Treating Fields (TTFields) therapy utilizes alternating electric fields to target
cancer cells directly.4 OncLive® spoke to Moshe Giladi, PhD; and Narasimha Kumar Karanam PhD, BCMAS; TTFields experts from Novocure®, to learn about the mechanism of action and the preclinical evidence in cancer cells, which shows enhanced effects when TTFields is used concomitantly with other modalities.
OncLive: How are electrical devices being used in medicine to date?
MOSHE GILADI, PhD: Electric fields of different frequencies have been used in medicine for many years in variety of applications and through
various modalities, among them microwave ablation, deep brain stimulation, and pacemakers.5-8 These devices may be external or implantable and may use continuous or pulsed electric fields with frequency-dependent effects.8
Electric fields applied at different frequencies can affect cell migration, cell proliferation, and apoptosis as well as other cellular functions depending on the magnitude of electricity used.9,10 Different magnitudes and frequencies of waves can interact differently with ions, cells, and bodily fluids and can affect cellular and tissue homeostasis mechanisms.11,12
OncLive: Is it possible to harness the power of electric fields to kill cancer cells?
GILADI: Ultimately, yes! Intermediate frequency electric waves offer a window of opportunity for cancer therapy that had not been explored until Prof Yoram Palti discovered the effect of TTFields, which, based on preclinical data, disrupt cellular processes critical for cancer cell viability and tumor progression, ultimately leading to cancer cell death.1,13,14 The essence of the invention is all about the tuning of the frequency to a range of several hundred kHz, which, for the first time, allows us to generate electric fields within cells without all the negative effects typically associated with electric fields. There is no electrification, no nerve or muscle stimulation, and no substantial heating. These fields do exactly what any other electric fields do — apply forces on charges.1,13,14
OncLive: What is TTFields therapy?
GILADI: Based on preclinical data, TTFields therapy is a noninvasive treatment modality applied through transducer arrays connected to an external, wearable, portable delivery system that has the potential to provide effective, selective, and nontoxic treatment.15 TTFields therapy is delivered locoregionally and is currently under investigation for use in patients with numerous solid tumor types.15
OncLive: How does TTFields therapy differ from existing medical devices in terms of frequency and intensity?
KARANAM: The effect of electric fields is dependent upon the frequency used.13 Very low frequencies (<1 kHz) result in membrane depolarization and can stimulate nerve, muscle, and heart tissues.13,14 Very high frequencies (above many MHz) can result in a heating or burning effect.14 However, intermediate frequencies (100-300 kHz) delivered at low intensity have an antiproliferative effect on dividing cancerous cells.13,14 TTFields are based on the generation of electric fields in frequencies between 100 to 500 kHz with alternating currents; the optimal frequency varies based on tumor type.1,4,13
OncLive: What enables TTFields therapy to selectively disrupt multiple cancer cell processes while sparing healthy cells?
GILADI: Based on preclinical data, TTFields spare healthy cells because these have different properties from cancer cells, including division rate, morphology, and electrical properties.13,16 TTFields induce an antimitotic effect, and cancer cells, having a very high division rate, are more affected. In addition, cancer cells look different from normal cells; they have different content, and their membrane potential is different.17 TTFields exploit unique electrical properties of cancer cells to target them, and, indeed, they were found to have a frequency-dependent effect on different cancer cells, thus contributing to the specificity and ability to spare normal cells (Figure 1).1,15 TTFields’ locoregional delivery and intensity-dependent effect contribute to their specificity, as tissue outside the treatment region is not exposed to high enough electric field intensities to be affected.1,4,13,15
FIGURE 1
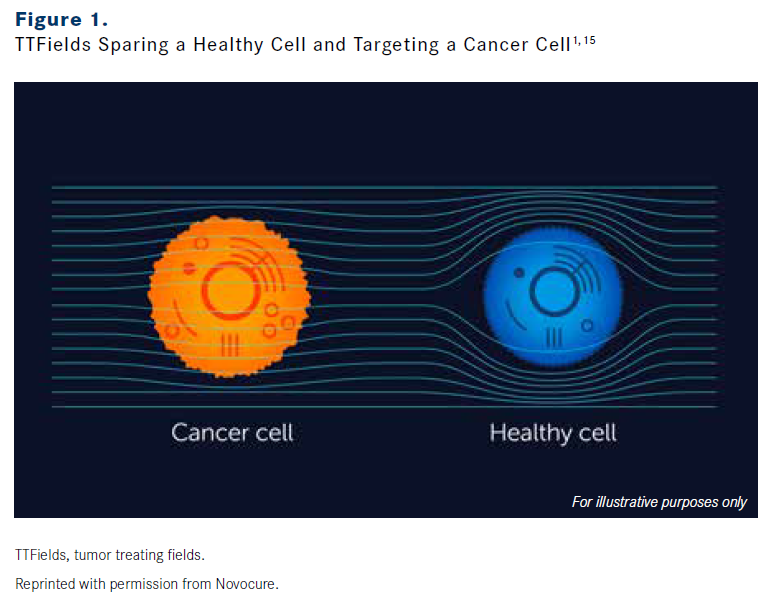
OncLive: How does TTFields therapy induce an antimitotic effect?
KARANAM: Tubulins, the building blocks of microtubules that aid in chromosome segregation during mitosis, possess very high dipole moments.1,18 Based on preclinical data, by inducing tubulin dipole alignment, TTFields interfere with the polymerization of microtubules (Figure 2).1,18 Septins, which are normally located in the mitotic furrow, also have high dipole moments, and their localization is also influenced by dipole alignment.13,19 In cells that are able to proceed from metaphase to cytokinesis, the hourglass shape formed result in nonuniform electric fields and dielectrophoretic forces. TTFields-induced mitotic errors lead to chromosome missegregation and multinucleated and micronucleated daughter cells.1,13,15,19,20
FIGURE 2
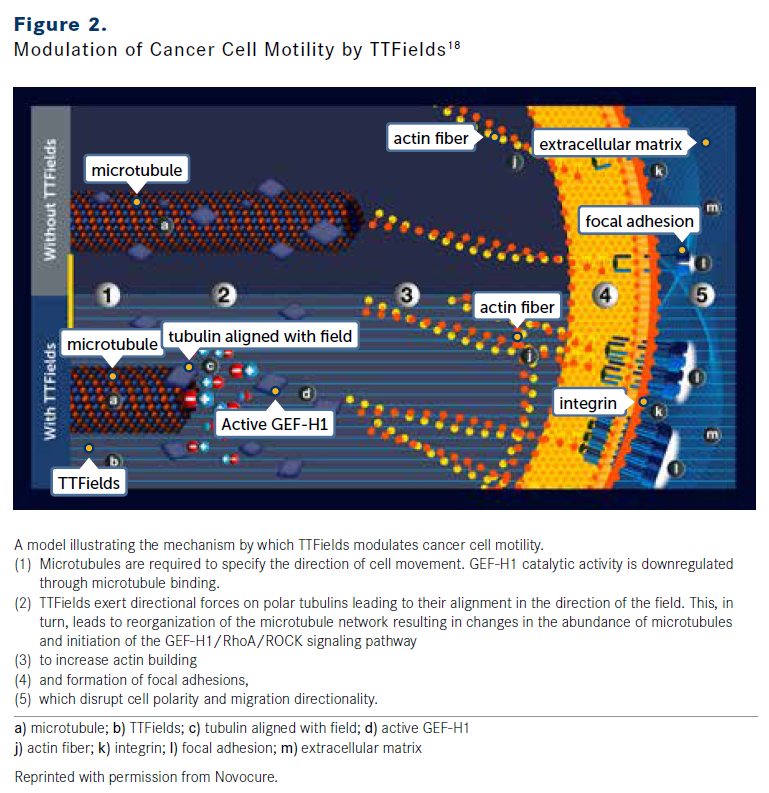
OncLive: How does the antimitotic effect of TTFields therapy lead to a downstream activation of the immune system?
GILADI: Based on preclinical data, immunogenic cell death is a downstream effect of the above-mentioned disruption of the mitotic process and the creation of aneuploid daughter cells (Figure 3).20,21 It has been shown that aneuploidy can lead to endoplasmic reticulum (ER) stress caused by disruptions in homeostasis.22 As shown in multiple in vitro models, the release of the alarmin high-mobility group box 1 (HMGB1) from the nucleus of the dying cell leads to an enhanced maturation of antigen presenting cells (APCs), and the exposure of cell surface calreticulin induces antigen uptake by the APCs (Figure 4).13,21 Recently, the increased levels of HMGB1 were also shown to be present in an in vivo model, supporting its role in the immune-mediated death process.23
Once the APCs have ingested the antigens from the dead and dying tumor cells, they travel through the lymphatic system and present their load to T cells, leading to T-cell activation and proliferation of clones with the ability to kill the tumor cells by recognizing their specific antigens.24
FIGURE 3

FIGURE 4
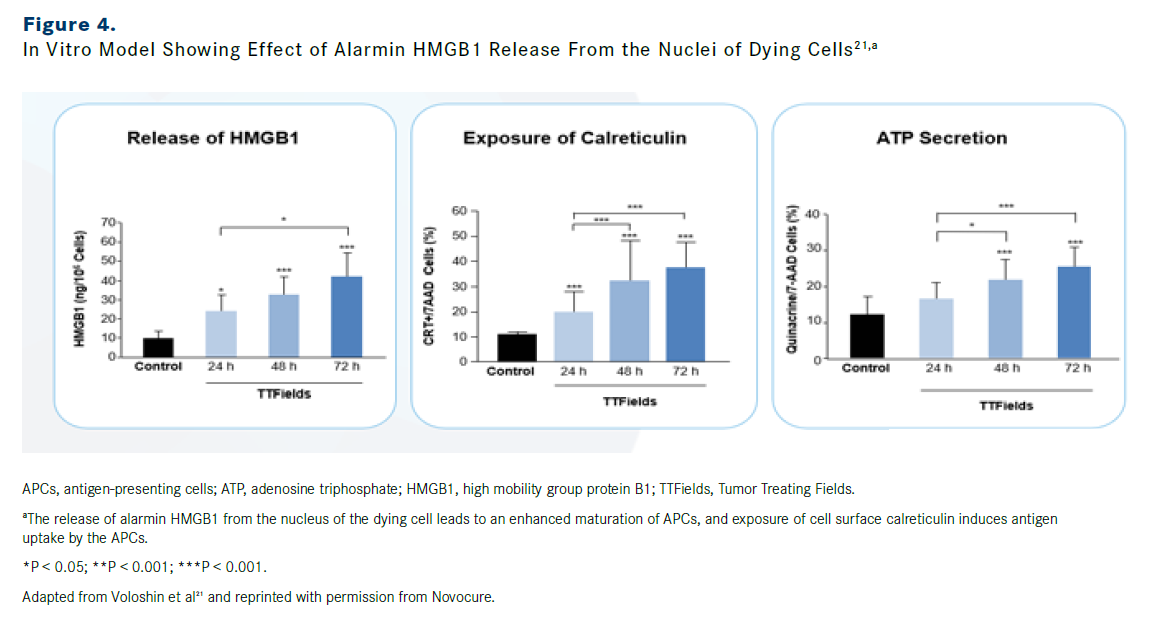
OncLive: What additional effects does TTFields therapy have on the immune system?
GILADI: In preclinical data, researchers found that cancer cells treated with TTFields have large clusters of micronuclei in the cytosol, thus activating 2 major cytosolic DNA sensors (cyclic GMP-AMP synthase [cGAS] and absent in melanoma 2 [AIM2]) and their cognate cGAS/stimulator of interferon genes (STINGs) and AIM2/caspase 1 inflammasomes, which produce type 1 interferons (T1IFNs), genes that respond to T1IFNs, and proinflammatory cytokines.25
OncLive: How can the immune effects of TTFields therapy be combined with those of other agents?
KARANAM: The immune system is normally tightly controlled to prevent the kind of overreaction that is seen in autoimmune diseases.23 Tumors have the capacity to evade the control of the immune system through the inhibitory actions of immune checkpoints such as the cytotoxic T-lymphocyte–associated protein 4 (CTLA-4).26
Preclinical evidence suggests that combining TTFields with immune checkpoint inhibitors (ICI) designed to stimulate an immune response has the potential for enhanced effects. TTFields used with PD-1, PD-L1, and CTLA4 inhibition in mouse models, have demonstrated decreased tumor size, increased immune cell infiltration, and increased cytokine production (eg, interferon-γ) versus monotherapy alone as assessed in tumor models.21,23
OncLive: What are the effects of TTFields on the DNA damage repair pathway and replication stress?
KARANAM: In preclinical data, DNA repair mechanisms are impaired by TTFields application through the downregulation of certain genes, such as BRCA1, that are vital for a coordinated repair mechanism, thus possibly increasing the efficacy of drug or radiation therapy.27,28 Through downregulation of Fanconi anemia (FA) pathway genes and MCM gene expression, TTFields increase replication stress in dividing cancer cells.13,29 The hallmarks of cancer cells include mild levels of DNA damage repair impairment and replication stress that maintain genomic instability. As a result of TTFields treatment, DNA damage and replication stress are elevated to lethal levels, ultimately resulting in cell death.13,29
OncLive: How about taking advantage of the impaired DNA repair mechanism?
KARANAM: The concomitant application of TTFields with other agents causing DNA damage is another promising approach. Based on preclinical data, TTFields therapy downregulates the BRCA/FA pathway, resulting in enhanced sensitivity to agents such as cisplatin and pemetrexed as shown both in vitro and in vivo (animal model).30 The resulting state induced by TTFields results in synthetic lethality in conjunction with PARP inhibition.29,31
Another notable mechanism for DNA repair is the promoter methylation of O6-methylguanine-DNA methyltransferase (MGMT), an enzyme that prevents the expression of the MGMT repair gene. This is especially important in glioblastoma, since resistance to the common chemotherapeutic agent temozolomide (TMZ) is associated with the presence of unmethylated tumors with increased MGMT activity.32
TTFields therapy enhances the effectiveness of TMZ in glioblastoma cell lines, irrespective of MGMT expression levels.33
OncLive: What are the effects in concomitant application of radiotherapy (RT) and TTFields application?
KARANAM: The concomitant application of RT with TTFields therapy has been evaluated as well in preclinical data. In a variety of in vitro models, it was demonstrated that TTFields exposure increased sensitivity of cells to RT.27,28,34 An even stronger effect was demonstrated in cells if TTFields therapy was applied before RT.28
OncLive: Could the potential convergence of these multimodal mechanisms enhance the effect of the TTFields?
GILADI: Preclinical evidence in cancer cells shows enhanced effects when TTFields is used concomitantly with other modalities. Due to its multi mechanistic actions, TTFields therapy can be added to cancer treatment modalities in approved indications and has demonstrated enhanced effects across solid tumor types when used with chemotherapy, RT, ICIs (PD-1, PD-L1), or PARP inhibition in preclinical models.4,21,24,29
Summary
TTFields therapy provides clinical versatility that has potential to help address treatment challenges across a range of solid tumors. TTFields are electric fields that exert physical forces to kill cancer cells via a variety of mechanisms while sparing healthy cells. The multiple, distinct mechanisms of TTFields therapy work together to selectively target and kill cancer cells. Due to its multiple mechanistic actions, TTFields therapy can be added to cancer treatment modalities in approved indications. As a highly versatile first-in-class modality, TTFields therapy has significant potential for broad applicability across solid tumor types and lines of therapy.1,2,13,15,24
To learn more about TTFields therapy, visit www.tumortreatingfieldstherapy.com.
References
1. Kirson ED et al. Proc Natl Acad Sci U S A. 2007;104(24):10152-10157.
2. Giladi M et al. Sci Rep. 2015;5:18046.
3. Siegel RL et al. CA Cancer J Clin. 2023;73(1):17-48.
4. Moser JC et al. Cancer Res. 2022;82(20):3650-3658.
5. Das R et al. Front Bioeng Biotechnol. 2022;9:795300.
6. Krauss JK et al. Nat Rev Neurol. 2021;17(2):75-87.
7. Mulpuru SK et al. J Am Coll Cardiol. 2017;69(2):189-210.
8. Zhao S et al. Cell Mol Life Sci. 2020;77(14):2681-2699.
9. Love MR et al. J Cell Physiol. 2018;233(3):1860-1876.
10. Szewczyk PK et al. ACS Biomater Sci Eng. 2019;5(2):582-593.
11. Hieda I et al. Int J Antennas Propag. 2013;2013(4):305362:1-6.
12. Song B et al. Proc Natl Acad Sci U S A. 2002;99(21):13577-13582.
13. Karanam NK et al. Int J Radiat Biol. 2021;97(8):1044-1054.
14. Kirson ED et al. Cancer Res. 2004;64(9):3288-3295.
15. Mun EJ et al. Clin Cancer Res. 2018;24(2):266-275.
16. Al Ahmad M et al. IEEE Access. 2018;6:25979-25986.
17. Runel G et al. Cells. 2021;10(4):887.
18. Voloshin T et al. Cancers (Basel). 2020;12(10):3016.
19. Gera N et al. PLoS One. 2015;10(5):e0125269.
20. Shteingauz A et al. Cell Death Dis. 2018;9(11):1074.
21. Voloshin T et al. Cancer Immunol Immunother. 2020;69(7):1191-1204.
22. Ohashi A et al. Nat Commun. 2015;6:7668.
23. Barsheshet Y et al. Int J Mol Sci. 2022;23(22):14073.
24. Rominiyi O et al. Br J Cancer. 2021;124(4):697-709.
25. Chen D et al. J Clin Invest. 2022;132(8):e149258.
26. Brunner-Weinzierl MC et al. Front Immunol. 2018;9:2737.
27. Giladi M et al. Radiat Oncol. 2017;12(1):206.
28. Karanam NK et al. Cell Death Dis. 2017;8(3):e2711.
29. Karanam NK et al. Transl Res. 2020;217:33-46.
30. Mumblat H et al. Lung Cancer. 2021;160:99-110.
31. Martinez-Conde A et al. Int J Radiat Oncol Biol Phys. 2022;114(3):e276.
32. Singh N et al. Cancer Drug Resist. 2021;4(1):17-43.
33. Fishman H et al. J Neurooncol. 2023;163(1):83-94.
34. Kim EH et al. Oncotarget. 2016;7(38):62267-62279.
©2024 Novocure GmbH. All Rights Reserved. Novocure is a registered trademark of Novocure GmbH. US-NOV-0377 v1.0. April 2024.