Opening the Door for Novel Therapies in Triple-Negative Breast Cancer
During a recent OncLive Peer Exchange®, the expert panel focused on the development of immunotherapy and other notable successes based on recent presentations at the 2019 San Antonio Breast Cancer Symposium.
Debu Tripathy, MD, Professor and Chairman Department of Breast Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center

Debu Tripathy, MD
A wave of change has taken hold of the treatment landscape for triple-negative breast cancer (TNBC). Recent successes demonstrated in clinical trial data have led to the approval of new immunotherapy and targeted agents, thanks in part to the molecular classification of TNBC.
“Triple-negative breast cancer remains a very challenging area,” said Debu Tripathy, MD, who served as moderator for a recent OncLive Peer Exchange®. The expert panel focused on the development of immunotherapy and other notable successes based on recent presentations at the 2019 San Antonio Breast Cancer Symposium.
Checkpoint Inhibitors
PD-L1 Evaluation
PD-L1 status plays an important role in firstline treatment decisions for patients with TNBCs that are PD-L1—positive and it appears to be the best predictor of patient response to immune checkpoint inhibitors at this time. In determining whether a patient with TNBC would be an appropriate candidate for immunotherapeutic agents, the panelists agree that early PD-L1 testing is needed prior to initiating first-line treatment. However, “the determination of PD-L1 positivity has been reasonably controversial because there are a number of tests depending on the specific immune checkpoint inhibitor,” said Hope S. Rugo, MD, FASCO.
Currently, the SP142 (VENTANA PD-L1) immunohistochemical assay is the only approved PD-L1 companion diagnostic for TNBC, with ≥1% positivity needed for treatment with atezolizumab (Tecentriq) in combination with nab-paclitaxel (Abraxane).1-3
The panelists look forward to results from ongoing trials of PD-1/PD-L1 inhibitors that should provide more data on PD-L1 testing. “What we found was that PD-L1 still seemed to be the best determinant of response to atezolizumab… but I think we’re going to see a lot more data in the future,” said Rugo.
Once first-line treatment has begun, next-generation sequencing is increasingly important for management decisions. “I think there’s now evolving data for some of these, albeit rare, mutations, that could give us [a] potential target…. We should be doing next-generation sequencing in addition to PD-L1 testing,” said Ian E. Krop, MD, PhD. Panelists expressed interest in next-generation sequencing to help identify patients with TNBCs with AKT or NKRT alterations that could be targeted with specific therapeutic agents.
IMpassion130 Trial
The SP142 assay was validated in the phase III IMpower130 trial (NCT02367781) that enrolled 902 patients with untreated metastatic TNBC and randomized patients to receive nab-paclitaxel with either atezolizumab or placebo. Patients continued treatment until disease progression or unacceptable toxicity. IMpassion130 trial end points were progression-free survival (PFS) and overall survival (OS) based on intention-to-treat (ITT) analyses.4
The median PFS was 7.2 months in the atezolizumab group compared with 5.5 months in the placebo group (HR for progression or death, 0.80; 95% CI, 0.69-0.92). For patients with PD-L1-positive tumors, median PFS was 7.5 months in the atezolizumab group compared with 5.0 months in the placebo group (HR, 0.62; 95% CI, 0.49-0.78).4
At the time of first interim analysis of the ITT population, the median OS was 21.3 months in the atezolizumab group compared with 17.6 months in the placebo group (HR for death, 0.84; 95% CI, 0.69-1.02; P = .08). For those patients with PD-L1-positive tumors, the median OS was 25.0 months in the atezolizumab group compared with 15.5 months in the placebo group (HR, 0.62; 95% CI, 0.45-0.86).4
Tripathy said that the wealth of PD-L1 biomarker data in IMpassion130 was “tremendously helpful.” The initial IMpassion130 trial results ultimately led to the approval of atezolizumab for use in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic TNBC whose tumors express PD-L1 ≥1%.2
However, the secondary interim analysis for the ITT population in IMpassion130 showed that median OS did not differ significantly between the atezolizumab and placebo groups (21.0 and 18.7 months, respectively; HR, 0.86; 95% CI, 0.72-1.02; P = .078) in the ITT population. Investigators noted that the exploratory comparison among patients with PD-L1-positive tumors showed a “clinically meaningful” OS benefit (25 vs 18 months; stratified HR, 0.71; 0.54-0.94).5
Serious adverse events (AEs) included neutropenia and peripheral neuropathy. In the IMpassion130 trial, there were 3 treatmentrelated deaths (2 in the treatment group and 1 in the placebo group).5 “We see some life-threatening toxicity and I say to people the key thing is any organ can be affected,” said Rugo. “If you see something funny and it doesn’t go away and you hadn’t seen it before, it’s immune-related, and so you should treat it.”
Antibody-Drug Conjugates
Another exciting area that the panelists discussed was the progress in treating metastatic TNBC with antibody-drug conjugates (ADCs). “We’re making big advances in various agents. But I think the ADCs are, to me, an exciting new area of investigation,” said Rugo.
Targeted therapy also proved to be an area of great interest among the panelists. TROP-2 is overexpressed in 80% of patients with TNBC. For patients with metastatic disease “the median survivals are less than a year and the median PFS for most agents is in the range of 2 to 3 months,” said Adam M. Brufsky, MD, PhD.
Targeting TROP2 could afford better options for those patients with refractory metastatic TNBC, and efforts have begun with the agent sacituzumab govitecan.
Sacituzumab Govitecan
A total of 108 patients with metastatic TNBC who had received at least 2 previous anticancer therapies were enrolled into a phase I/II trial (NCT01631552) to receive sacituzumab govitecan until disease progression or unacceptable toxicity. The end points are objective response rate, duration of response, clinical benefit rate, PFS, and OS.6
The response rate was 34.3% and the median response duration was 9.1 months as assessed by independent central review. The clinical benefit rate was 45.4%. Median PFS and OS were 5.5 months and 13 months, respectively. With regard to AEs, 9.3% of patients experienced febrile neutropenia.6
“The overall survival clearly looked to be better than historical controls,” said Brufsky. Results from this trial led to the FDA accepting a new biologics license application for sacituzumab govitecan in December 2019 with an action date set for June 2, 2020. This follows an unsuccessful first outing for the agent, for which the FDA issued a complete response letter citing chemistry, manufacturing, and control matters as their primary concerns.7,8
The phase I/II results were impressive enough that there is speculation about whether, “the FDA [will] give the priority accelerated approval now or will they wait for the phase III. That’s the big question I think that everybody has in mind,” said Brufsky.
The phase III ASCENT trial (NCT02574455) has finished accrual in the same patient population and disease state and will randomize 529 participants to either sacituzumab govitecan or physician’s choice chemotherapy.
“I think it would be great to see data from ASCENT [in 2020]. What’s interesting about this particular drug is that sacituzumab govitecan has an SN38, which is the active metabolite of irinotecan,” noted Rugo, who added that this reduces diarrhea as an AE but does not eliminate hair loss.
PARP Inhibitors
PARP inhibitors are another therapeutic strategy for patients with TNBC who harbor either germline or somatic BRCA mutations. These patients account for approximately 20% to 25% of the TNBC population. Although the panelists note that progress in this arena is not moving as quickly as it is in other areas, such as ovarian cancer, studies are under way to investigate the utility of PARP in this subset of patients.9
BROCADE3 Trial
In BROCADE3 (NCT02163694), 509 patients with BRCA-positive, previously treated metastatic TNBC were randomly assigned 2:1 to receive veliparib plus carboplatin/paclitaxel or placebo plus carboplatin/paclitaxel. Patients completing carboplatin/ paclitaxel treatment without disease progression received veliparib or placebo maintenance.10
Median PFS was 19.3 months for the veliparib group versus 13.5 months in the placebo group (HR, 0.70; 95% CI, 0.54-0.90). At 3 years, the PFS was 26% for the veliparib group versus 11% for the placebo group.10 “There was a really big improvement in progression-free survival in favor of a maintenance strategy,” said Joyce A. O’Shaughnessy, MD.
“The curves actually separated when you started the maintenance,” added Brufsky. “They didn’t separate until you started the maintenance.”
DORA Trial
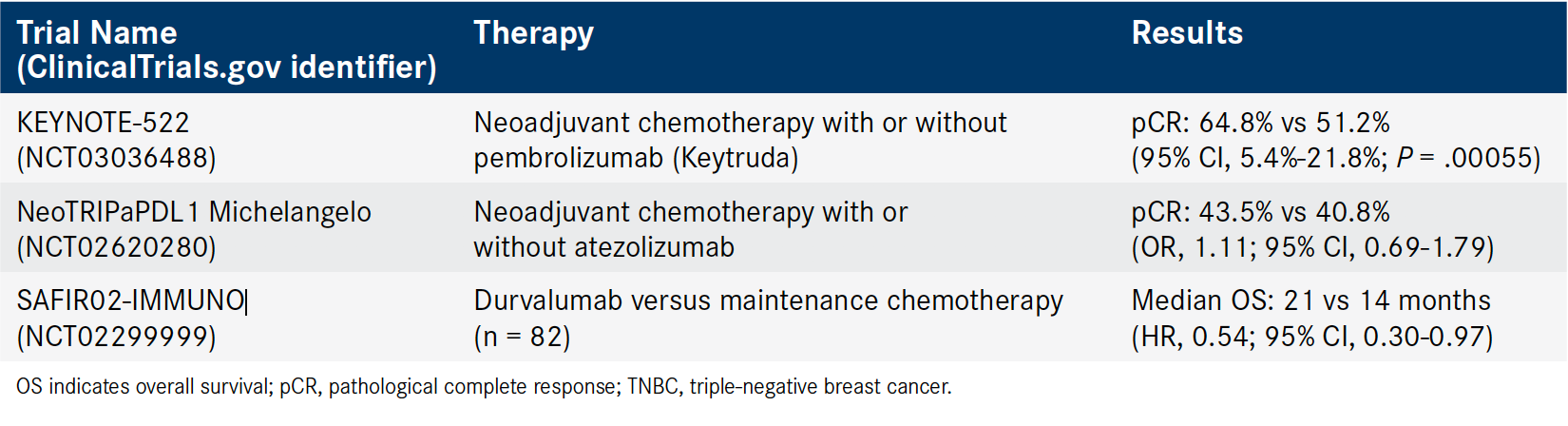
Table. Select Phase II/III Neoadjuvant Immunotherapy Trials in TNBC12-14 (Click to Enlarge)
I think the DORA trial, which is being conducted in triple-negative breast cancer, is really interesting,” said O’Shaughnessy. “Right now, we don’t have enough data to combine a PARP inhibitor with the checkpoint inhibitor in the first line. I think if a patient is germline-BRCA mutant and her cancer is PD-L1-positive, I will go where I know there’s a survival advantage in a phase III trial.”
Neoadjuvant Therapy
Panelists also focused on neoadjuvant therapy in TNBC. “There [have] been a lot of data now coming out from using immunotherapy in that setting with different checkpoint inhibitors,” Tripathy said (Table12-14).
KEYNOTE-522
The FDA granted pembrolizumab (Keytruda) plus neoadjuvant chemotherapy a breakthrough therapy designation for patients with early-stage TNBC after demonstrating manageable safety and antitumor activity in the KEYNOTE-173 and I-SPY2 trials. Investigators are further exploring the anti— PD-1 neoadjuvant chemotherapy combination in the KEYNOTE-522 (NCT03036488) trial.
The phase III trial randomized 1174 patients with early-stage TNBC 2:1 to receive neoadjuvant chemotherapy with or without pembrolizumab following definitive surgery. Patients continued treatment until recurrence or toxicity, and the primary end points were pathological complete response (pCR) and event-free survival (EFS).
Among patients whose cancers had lymph node involvement, the addition of pembrolizumab was associated with a 64.8% pCR rate compared with a 51.2% pCR rate in the placebo group.12
The KEYNOTE-522 findings showed “one of the highest pCR rates ever reported in a triple-negative population and a big population,” said Rugo. However, the panelists are looking forward to the full results of KEYNOTE-522 and other trials that “are powered by event-free survival, [which] should be our standard,” Rugo added. At the time of analysis, a favorable trend for EFS was observed in the pembrolizumab arm (HR, 0.63).12
NeoTRIPaPDL1 MICHELANGELO Trial
In NeoTRIPaPDL1 Michelangelo (NCT02620280), 280 patients with TNBC were randomized to receive neoadjuvant chemotherapy with or without atezolizumab. Patients then underwent surgery before receiving adjuvant chemotherapy. The primary end point was EFS at 5 years after randomization of the final patient. The rate of pCR was a secondary end point.
The addition of atezolizumab was associated with a 43.5% pCR rate compared with 40.8% in the chemotherapy-only group (OR, 1.11; 95% CI, 0.69-1.79). Results also showed that PD-L1 expression (≥1%) was the most significant factor that influenced pCR rate with atezolizumab over chemotherapy alone (OR, 2.08; 95% CI, 1.64-2.65). Data for EFS are not yet available.13
Panelists discussed the somewhat disappointing results from this much smaller neoadjuvant study. “The pCR rates were not better with atezolizumab, which was kind of surprising given this big difference in [the] KEYNOTE-522 trial…. I have the bias that it’s not agent-specific, but it is more that you generate a lot of immunity from the host by giving an anthracycline with cyclophosphamide. Maybe you don’t need the cyclophosphamide,” said Rugo. “Is it possible that part of the difference between the 2 trials was the duration…did they have enough time to generate a response?” asked Krop.
SAFIR02 Trial
The panelists also discussed SAFIR02- IMMUNO, a substudy of the SAFIR02_Breast trial (NCT02299999). In this phase II trial, patients with HER2-negative metastatic breast cancer who were eligible for or were currently being treated with first- or second-line chemotherapy underwent genomic evaluation. Those with preselected genomic alterations for which a targeted therapy was available were placed in a substudy of targeted therapy versus maintenance chemotherapy.
Patients with no targetable genetic alterations (n = 190) were randomized 2:1 to receive either durvalumab or maintenance chemotherapy. For 82 patients with TNBC, the median OS was 21 months with durvalumab compared with 14 months for chemotherapy (HR, 0.54; 95% CI, 0.30-0.97). Researchers also stratified results for patients with metastatic breast cancer (not only TNBC) whose tumors were PD-L1 positive (≥1%). Among the 44 patients with PD-L1- positive tumors, the median OS was 26 months for the durvalumab group compared with 12 months for the chemotherapy group (HR, 0.42; 95% CI, 0.17-1.05).14
The panelists were encouraged by both the TNBC findings and PD-L1 group findings, despite the latter not reaching statistical significance. Their discussion of why the trial failed to find a difference in the PD-L1 substudy focused on the small study size and the need for longer follow-up. “Maybe we need to give it in a different way or select the right group of patients. For example, if you took the responders to checkpoint inhibition, maybe it would have made a difference,” said Rugo.
Looking Ahead
The recent increase in clinical trials and the options afforded to patients with TNBC point toward positive movement in the field. “We’re making big advances in various agents,” said Rugo. “The good news is that we have a lot of trials in this space. There’s been a lot of interest from the community in subscribing patients to these trials,” said Tripathy.
However, these advances also come with challenges. “I think this area is going to be really complicated by the fact that we’ve got a lot of trials and the events are going to be small. They’re not really, with the exception of some of the really large studies, powered to look at event-free survival,” added Tripathy.
References
- Ventana PD-L1 (SP142) assay. Roche Diagnostics website. diagnostics.roche.com/us/en/products/tests/ventana-pd-l1-_sp142-assay1.html. Updated February 17, 2020. Accessed February 17, 2020.
- Tecentriq [package insert]. South San Francisco, CA: Genentech Inc; 2019. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761034s021lbl.pdf. Updated December 2019. Accessed February 17, 2020.
- List of cleared or approved companion diagnostic devices (in vitro and imaging tools). FDA website. www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools. Updated January 8, 2020. Accessed February 17, 2020.
- Schmid P, Adams S, Rugo HS, et al; IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108-2121. doi: 10.1056/NEJMoa1809615.
- Schmid P, Rugo HS, Adams S, et al; IMpassion130 Trial Investigators. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44-59. doi: 10.1016/S1470-2045(19)30689-8.
- Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741-751. doi: 10.1056/NEJMoa1814213.
- Immunomedics announces FDA acceptance for filing of biologics license application resubmission for sacituzumab govitecan to treat metastatic triple-negative breast cancer [press release]. Morris Plains, NJ: Immunomedics, Inc; December 26, 2019. bit.ly/37iQ4ZD. Accessed February 17, 2020.
- Immunomedics receives complete response letter from fda for sacituzumab govitecan biologics license application [news release]. Morris Plains, NJ: Immunomedics, Inc; January 17, 2019. bit.ly/2sBcDGG. Accessed February 17, 2020.
- Papadimitriou M, Mountzios G, Papadimitriou CA. The role of PARP inhibition in triple-negative breast cancer: unraveling the wide spectrum of synthetic lethality. Cancer Treat Rev. 2018;67:34-44. doi: 10.1016/j.ctrv.2018.04.010.
- Dieras VC, Han HS, Kaufman B, et al. Phase III study of veliparib with carboplatin and paclitaxel in HER2-negative advanced/metastatic gBRCA-associated breast cancer. Ann Oncol. 2019;30(suppl 5; abstr LBA9):v857-v858. doi: 10.1093/annonc/mdz394.008.
- Sammons S, Tan TJY, Traina TA, et al. Dora: a randomized phase II multicenter maintenance study of olaparib alone or olaparib in combination with durvalumab in platinum responsive advanced triple-negative breast cancer (aTNBC. J Clin Oncol. 2019;37(suppl 15; abstr TPS1113). doi: 10.1200/JCO.2019.37.15_suppl.TPS1113.
- Schmid P, Cortés J, Dent R, et al. KEYNOTE-522: phase III study of pembrolizumab + chemotherapy vs placebo + chemotherapy as neoadjuvant treatment followed by pembrolizumab vs placebo as adjuvant treatment for triple-negative breast cancer (TNBC). Ann Oncol. 2019;30(suppl 5):v851-v934. doi: 10.1093/annonc/mdz394.
- Gianni L, Huang C-S, Egle D, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Presented at: San Antonio Breast Cancer Symposium; December 10-14, 2019; San Antonio, TX. Abstract GS3-04. bit.ly/2spCzYE.
- Dalenc F, Garberis I, Filleron T, et al. Durvalumab compared to maintenance chemotherapy in patients with metastatic breast cancer: results from phase II randomized trial SAFIR02-IMMUNO. Presented at: San Antonio Breast Cancer Symposium; December 10-14; San Antonio, TX. Abstract GS3-02. bit.ly/2Slnpyl.
One upcoming study that is generating interest among the panelists is the phase II DORA trial (NCT03167619). It is designed to explore the efficacy of olaparib with or without durvalumab (Imfinzi) as maintenance therapy for patients with metastatic TNBC who have demonstrated benefit with platinum-based chemotherapy. The primary end point will be PFS.11




