Those Eureka Moments Glisten: A Conversation With Robert A. Weinberg, PhD
When Robert A. Weinberg was a boy, he loved dissecting what was complicated and figuring out what made it work.

Robert A. Weinberg, PhD
When Robert A. Weinberg was a boy, he loved dissecting what was complicated and figuring out what made it work.
When he wasn’t taking apart electric trains, he was studying genealogy, tracing the branches of his family tree.
But during those years in Pittsburgh, Pennsylvania, Weinberg had no idea where that passion might lead him.
He attended the Massachusetts Institute of Technology (MIT) because friends of his parents had gone there. He pursued medicine because “in those days, young Jewish boys became doctors” and switched to biology when he made the alarming discovery that “doctors had to stay up all night taking care of patients.”
“I’m not a person who’s planned out his course in life,” said Weinberg, 68, a renowned oncology researcher whose work has changed the world’s understanding of cancer. “I just stumble from one steppingstone to the next.”
Through a combination of talent and circumstance, that series of stumbles has coalesced into a long and successful career for Weinberg, a member of the US National Academy of Sciences who was presented with the National Medal of Science by President Bill Clinton in 1997 and named Scientist of the Year by Discover magazine in 1982 .

Oncogene Research Blooms
Weinberg’s discovery of the first cellular oncogene in mammalian cells, Ras, provided researchers with a deeper understanding of cancer that helped pave the way for the growing tide of targeted cancer therapies. Weinberg and his colleagues made another landmark discovery when they became the first to figure out what makes a normal, dormant gene change into a virulent oncogene. His lab also discovered the tumor suppressor retinoblastoma protein, opening the door to the isolation and study of genes that prevent the onset of cancer.
In 2000, Weinberg shared his comprehensive insights into what makes cancer cells abnormal by co-writing the seminal paper “Hallmarks of Cancer,” the most cited Cell journal article of all time.
“I’m driven by trying to figure things out,” Weinberg said. “It’s an unrelenting drive leading to occasional ‘eureka’ moments, with a lot of grunt work in between.”
Weinberg is director of the Ludwig Center for Molecular Oncology at MIT, where he is also a professor of biology. He joined the staff of his alma mater in the early 1970s, when he became part of its newly formed Center for Cancer Research. Nearly a decade after that, Weinberg became a founding member of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, where his lab remains.
Although Weinberg says he hasn’t “touched a test tube in 30 years,” he attends scientific meetings nearly every day in his own lab or with other colleagues to discuss results, offer critiques, and suggest additional experiments. To support that work, he wades through a constant “blizzard of e-mails” and edits the manuscripts of the researchers who work in his lab.
Lately, Weinberg has devoted a lot of time to revising a work of which he’s very proud: his 2007 graduate-level textbook, The Biology of Cancer. He gives talks at about 25 scientific meetings each year, and at MIT he spends about 15% of his time teaching half a course in introductory biology for undergraduates, along with half a graduate course in cancer biology.
In front of the classroom, Weinberg likes to entertain his undergraduates by revealing that he got a “D” in introductory biology, a course he didn’t enjoy when he took it at MIT. And he passes on this wisdom: “Most of the young people in high school have the conviction that biology is all about memorizing facts, when, in fact, it’s a very logical science with a lot of interesting questions that remain unexplored. It’s a very intellectually challenging field.”
While Weinberg wasn’t excited by his initial coursework during his days as a biology student, he found himself in the right place at the right time.
“The revolution in microbiology was just erupting,” Weinberg said. “As a junior, I was exposed to the genetic code, which was just being deciphered. All of a sudden it got very interesting, so I became interested in a serious way in the new biology— molecular biology—[and] in taking apart complicated things into their component parts, just like taking apart old electric motors.”

Robert A. Weinberg at a Glance
- Is 68 years old.
- With his wife, Amy, has 2 children: Aron, 32, and Leah Rosa, 30. Neither is a scientist.
- Is the son of parents who escaped Nazi Germany in 1938. “It made me realize that one should take very little for granted, that things are much more fragile and vulnerable to change than one imagines.”
- Knew early on that he didn’t want to be a dentist like his father. “My father spent his life looking in people’s mouths, and his father was a horse dealer who spent his life, in turn, looking in the mouths of horses. I didn’t want to be looking into mouths anymore.”
- Was taught as a child that behaving decently, having a sense of humor, and being learned were important, but that making a lot of money was not.
- Shrugs off adversity with a saying of his mother’s: “No matter what you do, it’s wrong in someone’s eyes.”
- Built a cabin in New Hampshire and enjoys gardening there, along with “mucking around the woods in my overalls.”
- Cites Star Wars as his favorite movie because “it was so mind-blowing when I saw it 30 years ago.”
- Has been the recipient of nearly 60 awards and honors for his work; has participated in 10 scientific committees, panels and symposia, in most cases as chairman; and has been a member of a dozen scientific advisory boards.
- Is “skeptical of those who go into cancer research to save humanity, because without the additional drive of finding it highly interesting, I don’t think one discovers that much. One needs to be motivated, in part, by some kind of burning curiosity.”
- Believes the “funding climate is very discouraging for young people going into basic biomedical research. Lots of funds are being put into multiteam collaborative efforts, which yield far less than the funding of individual labs.”
Pitching In for Civil Rights
Yet at the same time, another revolution was in progress, and it pulled Weinberg away from his studies. In 1965, after a year of graduate school, Weinberg left MIT to teach at Stillman College, a predominantly black institution in Tuscaloosa, Alabama. The city had just been ravaged by a hurricane, and the fight for desegregation was in full swing.
“I did feel I needed to do something for the world, and it was a time when people with my talents were needed,” said Weinberg. “I was the undergraduate biology department in that college that year. On weekends, I would buy sacks of fl our and rice and beans and carry them out to the tent cities of sharecroppers in Greene County, who’d been evicted from their land because they’d registered to vote.”
“Everybody was sure I’d stay in Alabama or end up getting buried under a dam, which had happened to some civil rights workers the year before,” Weinberg added. “But much to everyone’s surprise, I came back to MIT in 1966 and finished my PhD work.”
Weinberg went on to complete 2 postdoctoral fellowships: one with Ernest Winocour, PhD, at the Weizmann Institute of Science in Rehovot, Israel, where Weinberg enjoyed meeting and spending time with relatives; and the other with Renato Dulbecco, MD, at the Salk Institute for Biological Studies, in La Jolla, California, where Weinberg worked with the DNA tumor virus SV40 to study RNA metabolism.
MIT Opportunity Beckons
Weinberg was at the Salk Institute when he received a visit from his future. It came in the form of Salvador Luria, MD, a pioneer molecular biologist who was on staff at MIT.
“He told me I was going to be part of a new MIT cancer center he was founding,” recalled Weinberg. “He didn’t ask; he just told me. After a couple of days I said ‘OK,’ so I ended up coming back to MIT—not because I’d planned to do so. It just happened.”
In his first year at the cancer center, Weinberg worked as a research associate to David Baltimore, PhD, who had just discovered reverse transcriptase—a revelation that, 3 years later, would earn him the Nobel Prize. Baltimore shared it with 2 others, including Dulbecco. Intrigued by Baltimore’s work, Weinberg decided to explore retroviruses in his own lab.
“Around 1977, we became involved in studying viral oncogenes, and that led to us discover, in 1979, the first cellular oncogene in mammalian cells,” Weinberg said. “It was the most important thing that ever happened in my lab.”
The experiment involved taking genes from cells that had been exposed to a chemical carcinogen and putting those genes into normal cells. The result was that the normal cells became cancerous, proving for the first time that cancer is a genetic disease. The finding led to the discovery of additional oncogenes by other labs, opening the door to the possibility of targeted cancer therapies.
“It’s not as if somebody else would not have done it a year or two later,” a modest Weinberg said of his finding. “But that was my moment in the sun.”
Probing the Processes of Metastases
Latest Research:
In recent years, Professor Weinberg has been exploring the role of the epithelialmesenchymal transition (EMT) process in promoting the development of metastatic tumors. Weinberg discussed some of his theories during a June 5 plenary session lecture at the American Society of Clinical Oncology annual meeting, where he was honored with the Science of Oncology Award.
Here are excerpts from the noted researcher’s presentation, as well as answers to questions that OncLive posed:
- EMT is a complex, multifaceted program involving multiple changes in cell properties.
- EMT allows carcinoma cells to physically disseminate from primary tumors and, in the process, acquire stem cell properties that can initiate new tumors.
- Chemotherapeutic agents typically kill noncancer stem cells preferentially, creating “a dangerous situation because not only have these cells survived the initial onslaught of chemotherapy or radiotherapy but in addition they are capable of repopulating an entire tumor.”
“We will not succeed if we wipe out only the cancer stem cells, we will not succeed if we wipe out only the nonstem cells.” Weinberg said. “Both populations of cells need to be targeted in order to hope to achieve a durable clinical response given the plasticity and the bidirectional interconversion between these two different cell types.”
OncLive: YIn the lab, your m ost recent work has involved investigating how cancer cells spread, causing tumor recurrence. Can you explain more about these efforts?
Weinberg: Now that we have an understanding of how cancer cells can form primary tumors, we want to figure out how cancer kills people through the spread of primary tumors to distant sites in the body. Until recently, it’s been a mystery how cancer cells could acquire multiple powers to generate metastases.
Over the last five or 10 years, through a number of labs, including mine, we’ve learned that cancer cells do this by activating a dormant embryonic program in which normal cells migrate from one part of an embryo to another. The reactivation of this program, known as EMT, enables cancer cells to acquire a number of traits that, together, allow them to disseminate and spread to distant organ sites.
Clinical implications: Most currently used chemotherapeutics kill noncancer stem cells (CSCs) preferentially.
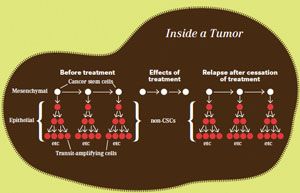
Adapted from Weinberg RA. The EMT and the pathogenesis of high-grade carcinomas. Presented at the American Society of Clinical Oncology Annual Meeting; June 5, 2011.
What role did your lab play in discovering this mechanism?
Although this was not our idea to begin with, it had never been examined experimentally. We did some of the first direct demonstrations of this as the case, and validated that this was a powerful idea. Two years ago, we reported that the product of forcing a cancer cell through an EMT program is a cancer stem cell that has the power to seed a new tumor. If you put it into a mouse, it can generate an entire tumor.
How will this work change the direction of cancer research?
This demonstrated that cancer stem cells are more resistant to chemotherapies than nonstem cells, so there are subpopulations of cancer cells in tumors that have greater or lesser sensitivity to being killed by anticancer therapeutics. This knowledge will allow the research community to generate new cancer stem cells that can then be tested for their susceptibility to being treated by existing cancer treatments.
Lab Work Consuming
The achievement didn’t escape the notice of Baltimore, who appointed Weinberg as a lab head when he founded the Whitehead Institute in 1982. It turned out to be a wise decision. During Weinberg’s first year at the Whitehead Institute, he experienced the biggest “aha” moment of his career.
He and his colleagues converted a normal gene into a cancer-causing bladder carcinoma oncogene, demonstrating for the first time how a healthy, dormant gene can be transformed into a virulent oncogene.
The cause of the cancer, Weinberg was fascinated to find, “was a point mutation, a single letter or base of DNA among the thousands of bases that, together, constitute a gene.”
Eight years later, Weinberg shared his comprehensive understanding in “The Hallmarks of Cancer,” which was co-authored by Douglas Hanahan, PhD. The article discussed “6 principles, or hallmarks, that turned out to be a very useful conceptualization that explains much of what makes cancer cells abnormal,” Weinberg said. He and Hanahan updated the article this year, adding information about more recently identified biological mechanisms that contribute to the creation of tumors.
In addition, Weinberg continues to unravel the mystery of cancer in his lab, where he and colleagues are researching the molecular mechanisms that control carcinoma progression and metastasis. Combined, his projects are all-encompassing— not so different, ironically, from the prospect of all-night patient care that discouraged him from pursuing a medical degree during his days as a student. Still, Weinberg has no intention of slowing his pace.
“I don’t have time to read a book or to listen to music, which I love doing, so that’s been a real sacrifice. It’s been going on for 40 years, and I can’t get them back,” the scientist said. “Still, I feel blessed. Ninety-five percent of the people in the world don’t enjoy what they’re doing, so my career has been a stroke of good fortune, a gift which I’ve never taken for granted.”
It’s a career whose legacy, Weinberg hopes, will reach far into the future—in particular, through the work of the talented younger scientists he’s mentored over the years.
“What I’m most proud of,” he said, “is the people I’ve trained. That kind of satisfaction continues to come back to me, and that’s all I can hope for.”
Beth Fand Incollingo is a New Jersey-based writer and editor, and owner of the communications firm Texterity, LLC.




