Diffusion-Weighted MRI Studies Explored at VCU Massey Cancer Center
The Department of Radiation Oncology at Virginia Commonwealth University Massey Cancer Center has long been at the forefront worldwide in the development of various radiation therapy technologies that are now considered standard and have become widely available.
Elisabeth Weiss, MD, and Geoffrey Hugo, PhD

Elisabeth Weiss, MD
Radiation Oncologist and Professor of Radiation Oncology
Geoffrey Hugo, PhD
Medical Physicist and Associate Professor of Radiation Oncology
The Department of Radiation Oncology at Virginia Commonwealth University Massey Cancer Center has long been at the forefront worldwide in the development of various radiation therapy technologies that are now considered standard and have become widely available. Among those advancements is four-dimensional computed tomography (4D CT), where Massey has been among the principal developers. Massey also has a long tradition of implementing newly developed technology into clinical practice, such as respiratory gating, audiovisual biofeedback for respiration regularization, and implantation of fiducial markers for improved visualization of tumors during radiation treatment. In addition, VCU was one of the first to apply stereotactic radiotherapy techniques for lung tumors and other locations outside the brain.
The current standard of care for inoperable locally advanced lung cancer is a combination of chemotherapy and radiotherapy. However, despite many efforts to improve radiotherapy, the 5-year survival rate is only about 20%. Radiation oncologists face many challenges throughout the treatment process. High-quality images with good resolution are essential for radiotherapy planning, but they are complicated to acquire. Defining the microscopic boundary of malignant tumors can be particularly difficult because tumors move during imaging as the patient breathes.
Setting New Standards
Even with high-quality imaging, there are many potential sources of error throughout several weeks of daily treatments. Variations in patient position, tumor movement during respiration, and tumor deformation all make reliably targeting tumors during daily treatments especially difficult.18F-fluorodeoxyglucose positron emission tomography (FDG-PET) is the current standard for functional imaging of lung tumors. FDG-PET is able to distinguish lung tumor volumes with active dHuisease from noncancerous conditions, since the rapidly metabolizing cancer cells absorb the injected FDG trace much more than healthy tissue. FDG-PET is therefore used prior to treatment to detect tumors and any potential tumor spread such as involved lymph nodes or metastases in other organs. The functional imaging feature also makes it helpful to determine changes in metabolic tumor activity as a response to therapy.
Unfortunately, FDG-PET also has several limitations. It has poor spatial resolution, the measured signal can be affected by the presence of inflammation, and the injection of the FDG tracer every time imaging is performed exposes the patient to additional radiation. New imaging techniques for lung cancer are urgently needed.
This is why we are conducting a pair of unique, clinical studies developed at Massey assessing the use of magnetic resonance imaging (MRI) for treatment planning purposes and to observe changes in tumor volumes and tumor activity during treatment. The first study (NCT01859338) compares MRI with conventional CT imaging. The second clinical trial (NCT02059889) compares MRI with FDG-PET imaging.
MRI may hold key advantages over FDG-PET while providing similar performance for detecting tumor and tumor deposits and for differentiating between benign and malignant tissue. MRI is typically more readily available than FDG-PET. It has excellent soft tissue visualization and higher image resolution, and it provides both morphological and functional imaging information.
While MRI has not typically been used in chest imaging due to artifacts originating from respiration and heartbeat, MRI technology has advanced recently. For example, strong gradients, multichannel imaging, cardiac pulsation- and respiration- triggered imaging, and fast pulse sequences have all increased its utility in radiation therapy planning and treatment assessment.
DW-MRI for Radiotherapy Planning
Importantly, in other cancers such as rectum and cervical cancers, diffusion-weighted MRI (DW-MRI) has been shown to be a promising biomarker of tumor response to therapy. Assessing lung cancer response with MRI is a major focus of our research. If DW-MRI is shown to provide similar advantages as a biomarker for lung cancer, it could enable personalized treatment plans that can be adapted during the treatment process according to early response findings.To understand the potential benefits of DW-MRI, it is important to understand how the technology works. While FDG-PET uses a radioactive trace injected into the patient to measure metabolic activity in tissue, DW-MRI measures water diffusion, quantified by the apparent diffusion coefficient (ADC), to determine the microstructural composition of tissue. Because the size and density of cells affect water diffusion, it can be especially useful for the functional imaging of dense tumors. Cancer cells have a higher cell density, larger nucleus to cytoplasm ratio and less extracellular space in comparison to healthy tissue, and this results in reduced water diffusion and a low ADC.
In comparison to FDG-PET, DW-MRI for lung cancer staging has been shown to have similar sensitivity—approximately 70% to 90%—and nearly identical specificity. However, MRI is more widely available and provides a clearer image of the tumor with higher spatial resolution and also excellent visualization of soft tissue structures that are not displayed at all in FDG-PET.
DW-MRI as a Functional Biomarker
However, there are challenges. One issue is that interpretation of morphological and functional MR images is rather subjective. This is where it is important to take a multidisciplinary approach and evaluate images in consensus with experienced radiologists. It can also be difficult for a physician to interpret the complicated visual data, but computer modeling and prediction algorithms can be used to simplify and automate the process. Additionally, careful quality assurance and measurement protocols are of vital importance in this context. Image density that drifts on a CT or MR scanner can mask changes in tissue density, so proper equipment maintenance and calibration is critical, including phantom testing.While previous clinical developments and associated research had a strong focus on improving geometrical precision and accurate treatment delivery, more recent projects investigate a personalized treatment approach that is based on the identification of biomarkers of tumor activity and predictors of radiotherapy response that will allow individualized dose prescription and treatment adaptation. We feel this is one of the most promising aspects of DW-MRI for lung cancer therapy. Because radiotherapy kills tumor cells, it increases the ability of water to penetrate the tissue. Water penetration causes a subsequent increase in ADC that can be used to assess a patient’s ongoing response to radiotherapy. Depending on the observed tumor response, the total radiation dose and the distribution of radiation dose in the tumor can be individually adjusted to account for the observed microstructural change in the tumor tissue.
One of the critical challenges for adapting treatment using feedback from images taken during therapy is identifying corresponding anatomy at different time points that might have been distorted by treatment-related physiological processes in the tumor.
Developing methods to track anatomy based on landmarks and the ability to accurately accumulate delivered dose over deforming anatomy is one of our major research goals that is closely related to the identification of predictive imaging markers. Applying appropriate respiration management techniques such as triggered image acquisition using breath hold techniques are necessary for reproducible image acquisition and meaningful image analysis.
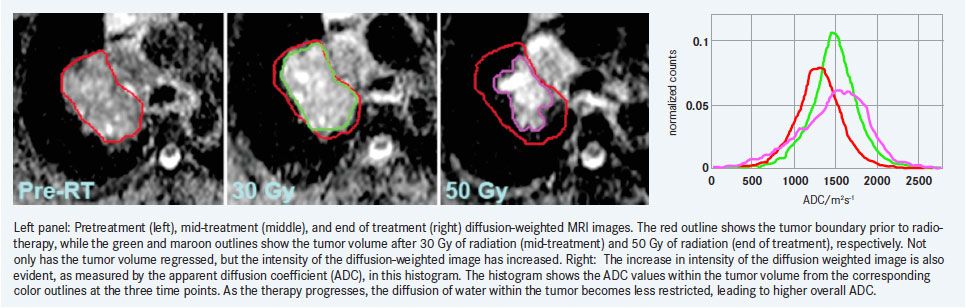
Initial results from our clinical research indicate significant increases in ADC values during treatment. In addition, these changes did not seem to correlate with volumetric tumor shrinkage, indicating that DW-MRI could be used as an independent marker of response.
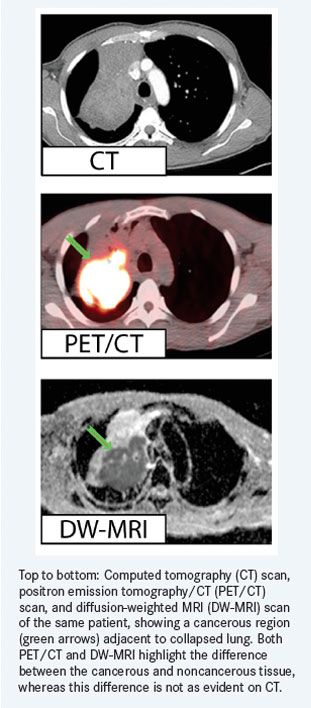
Early results from comparisons with CT images and FDG-PET images are encouraging regarding the differentiation of tumor from non-tumor tissue, which is important for defining the targets of radiation therapy. In particular, in collapsed lung situations where the tumor is “hidden” in dense lung tissue, MRI imaging supports the delineation of the tumor boundary, which is important for focused radiation dose delivery and sparing of surrounding sensitive tissues.
Future Steps
Another question we are hoping our research will answer is whether we can predict how well a patient will respond to radiotherapy treatment in the long term based on early changes in ADC value. It is still too premature to confirm the relationship, but our early observations have indicated that patients with small increases in ADC appear to have poor outcomes.Our multidisciplinary team at Massey will continue to research the potential for DW-MRI in radiation therapy for lung cancer. Further experiments are planned to clinically validate the use of DW-MRI as a biomarker for predicting tumor response, in particular against the current standard of FDG-PET.
The results from these studies will be used to develop standard protocols for the use of DW-MRI in the clinical setting. If successful, it could be a powerful new technique better allowing radiation oncologists to plan and adapt radiation therapy for the best possible outcomes for each individual patient.




