Kidney Cancer Genomic Studies Reveal New Targets and Complexities
Genome sequencing studies are beginning to match distinct genomic profiles to different subtypes of kidney cancer, driving a dramatic shift in our understanding of these diseases and how to treat them.
Kidney cancer is not a single disease, but a collection of histologically heterogeneous cancers that vary widely in their clinical course and response to treatment. Until recently, however, clinical trials have enrolled all types of patients and drugs have been broadly applied. Genome sequencing studies are beginning to match distinct genomic profiles to different subtypes, driving a dramatic shift in our understanding of these diseases and how to treat them.
Kidney Cancer’s Histologic Diversity
Researchers have uncovered substantial genomic heterogeneity, even within the same tumor, and have made important discoveries hinting at the important roles of chromatin modification and cancer cell metabolism in driving tumor formation. Although these malignancies present unique challenges, capitalizing on these findings could offer the potential for durable disease control and a brighter future.Kidney cancers account for about 4% of all adult malignancies. The most common form, renal cell carcinoma (RCC), can be further subdivided on the basis of histologic morphology into a number of different subtypes. The vast majority (60%-80%) are clear cell RCC (ccRCC), with the remainder broadly classed as non-ccRCC.
Targeting Key Drivers
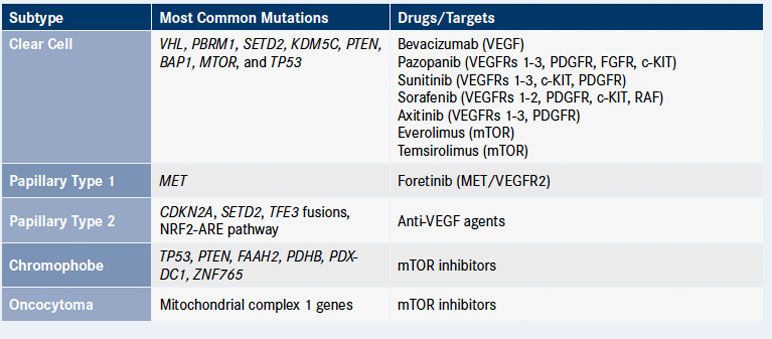
Further adding to the complexity, non-ccRCC tumors encompass additional histologic groups: papillary RCC (pRCC; 10%-15%), chromophobe RCC (5%), and collecting duct carcinoma (1%). More recently, rarer subtypes have been discovered, including translocation-linked, mucinous tubular, and spindle- type RCC in addition to tubulocystic carcinoma, collectively accounting for less than 1% of cases, but responsible for some particularly devastating outcomes. There also are typically benign epithelial tumors such as renal oncocytoma (5%), which can be difficult to distinguish from chromophobe RCC. All forms of kidney cancer have proved difficult to treat, notoriously resistant to chemotherapy, with surgical resection providing the only potentially curative option if patients can be identified at an early stage. Around 30% of patients present with metastatic disease, with a further one-third developing metastases after surgical resection.Genetically speaking, kidney cancers can be either inherited or sporadic. Although less than 10% of kidney cancer cases are considered to have an inherited basis, the study of the four major autosomal dominant inherited kidney cancer syndromes has been pivotal to our understanding of the development and progression of this disease. Indeed, the some of the major drivers of inherited syndromes are common to many sporadic cases. These syndromes are the von Hippel-Lindau (VHL) syndrome, hereditary leiomyomatosis and renal cell cancer (HLRCC), hereditary papillary renal cancer (HPRC), and Birt-Hogg-Dubé syndrome (BHD).
Mutations in the VHL gene that are behind the hereditary risk of kidney cancer in patients with that syndrome are the most common. More than 90% of cases of ccRCC, both inherited and sporadic, exhibit deregulation of the VHL pathway. The VHL gene encodes a protein that forms a central part of an E3 ubiquitin ligase complex. This complex “tags” target proteins in the cell with ubiquitin molecules that signal to the proteasomal machinery that the proteins need to be destroyed and removed from the cell. Among VHL’s target proteins are the hypoxia-inducible factors (HIFs), primarily HIF-1α, oxygen-sensing transcription factors that mediate the expression of a number of genes in the nucleus that have hallmark roles in carcinogenesis.
One such HIF-target gene is VEGF, which has a well-known role in regulating angiogenesis. Thus, in the presence of a defective VHL gene, HIF-1α is no longer disposed of as it should be, leading to its accumulation and corresponding increases in the expression of downstream genes like VEGF. As researchers have worked to dissect the molecular pathways of kidney carcinogenesis, it has been shown that increased expression of HIF-1α and VEGF is highly dependent on the upstream activity of another well-known oncogenic signaling network, the PI3K/mTOR pathway.
Drugs Approved for Kidney Cancer
Drugs targeting both the VEGF and PI3K/ mTOR pathways were already in development for other types of cancer and these discoveries paved the way for a decade of advancements in the treatment of metastatic RCC.The FDA has approved seven targeted therapies for kidney cancer since 2005. These include four multikinase inhibitors that inhibit the VEGF receptor (VEGFR) among other targets—sunitinib (Sutent), pazopanib (Votrient), sorafenib (Nexavar), and axitinib (Inlyta)—and the monoclonal antibody bevacizumab (Avastin). Two mTOR inhibitors, temsirolimus (Torisel) and everolimus (Afinitor), are approved as first- and second-line therapy, respectively.
Clear-Cell Renal Cell Carcinoma
Although these drugs provide an undeniable advance in progression-free survival (PFS) and other patient outcomes, only temsirolimus improved overall survival and none of these drugs has demonstrated long-term, durable remissions. Most recently, advances in the development of immunotherapy are showing promise for the treatment of metastatic RCC, with approval of the immune checkpoint inhibitor nivolumab (Opdivo) in the second-line setting in 2015. But not all patients respond to approved drugs and they provide no relief for patients with other, rarer forms of RCC.Beyond VHL, little was known about the genes involved in sporadic ccRCC, but exome sequencing has revealed a plethora of other key players at the genetic level. These include PBRM1, which encodes a subunit of the SWI/SNF chromatin remodeling complex, a group of proteins that act together to alter the way DNA is packaged: BAP1 (BRCA-associated protein 1), a histone deubiquitinase; and SETD2, a histone methyltransferase.
Intriguingly, these genes are located on the same region of chromosome 3p as VHL, suggesting that a single deletion in this chromosome could deal a devastating blow to renal cells and knock out one copy of four key tumor suppressors. All four genes are so-called “two-hit tumor suppressors,” meaning that a single assault on the gene is not enough to inactivate them. These observations have led to a proposed model of ccRCC carcinogenesis that begins with a VHL mutation. A subsequent chromosome 3p deletion strikes another blow to VHL and concomitantly impacts one copy of PBRM1, BAP1, and SETD2 (a phenomenon called loss of heterozygosity), rendering the tumors exquisitely sensitive to further alterations in these genes.
A Closer Look at Kidney Cancer
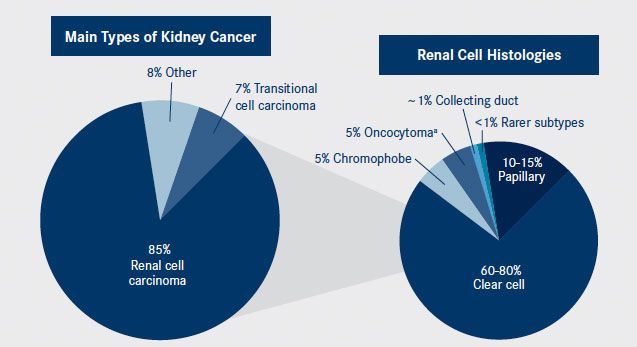
aOncocytomas are typically benign epithelial tumors that can be difficult to distinguish from chromophobe RCC.
Kidney cancer is a complex and heterogeneous disease. The main histologies, left pie, include renal cell, transitional cell, and other types such as Wilms tumors and renal sarcomas. The histology of renal cell carcinomas are further characterized into subtypes, right pie, including clear cell and several types of non—clear cell malignancies.
PBRM1 and BAP1 mutations tend to be mutually exclusive, with mutations in both occurring in less than 5% of cases, which led to suggestions that BAP1-mutant and PBRM1-mutant ccRCC represent two distinct classes of disease. Further studies have shown that BAP1, SETD2, and PBRM1 mutations are found in tumors with very different biology, potentially driving different patient outcomes. BAP1 mutations correlate with poor survival, while patients with PBRM1 mutations tend to fare better.
This laid the foundation for the first molecular classification of ccRCC, which could eventually be translated into clinical practice. Four molecular subtypes are proposed, which are associated with different outcomes. Patients with BAP1 and PBRM1 wild-type tumors have the best outcomes, followed by patients with tumors deficient in PBRM1 and then BAP1-deficient tumors. Finally, loss of both PBRM1 and BAP1, though rare, predicts the worst outcome.
In 2013, The Cancer Genome Atlas (TCGA) research network published a large-scale molecular classification of 500 primary nephrectomy samples from patients with ccRCC and matched normal pairs. They confirmed that the VHL/HIF pathway is a major player in this disease subtype and solidified the emerging role of epigenetic reprogramming, through SETD2, PBRM1, and other genes, as a key feature. When chromosome arm-level alterations were considered, there was a loss of chromosome 3p in more than 90% of cases, which encompasses the four frequently mutated tumor suppressor genes described above.
Non—Clear Cell Renal Cell Carcinoma
Through whole-exome sequencing the TCGA study identified 19 significantly mutated genes; besides those already mentioned, the eight most common genes included KDM5C, PTEN, MTOR, and TP53, implicating yet more tumor suppressors (PTEN and TP53) and epigenetic regulators (KDM5C), while highlighting the important role of the PI3K/mTOR pathway.More recently, researchers have turned their attention to non-ccRCC, about which significantly less was known. Studies have evaluated the group as a whole. For example, an analysis of 167 primary human tumors spanning renal oncocytomas, and papillary, chromophobe, and translocation RCC, among others, was recently published. This study identified 10 significantly mutated genes in pRCC, including MET, NF2, and SLC5A3, while in chromophobe RCC, TP53, PTEN, FAAH2, and PDHB mutations were among the most common alterations. Gene expression analysis identified a 5-gene set that could be used to stratify non-ccRCC in the clinic.
Others have examined individual non-ccRCC subtypes, such as the very recent study from the TCGA research network that provided a comprehensive molecular characterization of more than 150 primary pRCCs.
pRCC is commonly classed as type 1 and type 2 based on histological differences. This study confirmed that these tumors can also be defined by unique genomic characteristics. Alterations in the MET gene have been frequently observed in patients with pRCC, and in this study MET alterations were characteristic of type 1 pRCC, observed in 81% of samples. This suggests that pRCC may be sensitive to MET inhibition and clinical evaluation is ongoing.
The results of a phase II study of foretinib, an oral multikinase inhibitor that targets MET, demonstrated an overall response rate of 13.5%, in 74 patients with pRCC, with median PFS of 9.3 months, and the drug was well tolerated. A subgroup analysis found that patients with MET mutations had particularly good responses. Clinical trials of the MET inhibitor INC280 are also underway in patients with pRCC (NCT02019693).
Loss of the tumor suppressor CDKN2A was a characteristic of type 2 pRCC and was associated with significantly poorer patient outcomes, but in general this subtype was much more heterogeneous and shared few common driver mutations with type 1. Type 2 pRCC could be further subdivided into at least three subgroups, the most distinct of which were those defined by CpG island methylator phenotype (CIMP), in which there are high levels of promoter methylation that drive aberrant gene expression.
Mutation Landscape in Kidney Cancers
Heterogeneity Beyond Histology
Molecular characterization of 66 samples of chromophobe RCC, a much rarer form of RCC that is associated with germline mutations in the folliculin (FLCN) gene in Birt-Hogg Dubé syndrome and in the PTEN tumor suppressor gene in Cowden syndrome, identified a much lower mutational burden in this type of RCC, with only two significantly mutated genes, PTEN and TP53.Genome sequencing studies have illustrated the complexity and heterogeneity of kidney cancers, not only between the different subtypes, but also within a single tumor. Several groups undertook multiregion sequencing to examine mutation profiles in different parts of the same tumor. In ccRCC, mutations in VHL and, to a lesser degree, PBRM1 were found at a majority of sites within the same tumor; the researchers dubbed these truncal mutations. Other driver mutations such as BAP1 and SETD2 were found only in branch segments of the tumor and different portions of the same tumor had unique complements of branch mutations.
New Metabolic Targets
The researchers hypothesized that the truncal mutations reflect those acquired early in tumorigenesis, while those acquired at later stages are branch mutations. This could have important implications for therapeutic development, since targeting branch mutations may be significantly less effective than targeting truncal mutations. Studies in pRCC found that similar high-frequency mutations were not present in these tumors. Instead, truncal events appeared to involve chromosomal-scale copy number changes, particularly in chromosome 7, 12, 16, and 17 representing an unusual mechanism of tumorigenesis.It is increasingly recognized that cancer cells have a unique metabolism that helps them to thrive in their microenvironment. The best-characterized metabolic change is the Warburg effect, in which cancer cells display alterations in key metabolic genes that orchestrate a metabolic switch.
There were already hints of this from studies of hereditary pRCC, in which the FH gene, which encodes a key enzyme of the Krebs cycle, is frequently mutated. FH-deficient tumors display a Warburg-like metabolic shift. Molecular characterization studies of pRCC also noted frequent upregulation of the NRF2-antioxidant response element (ARE) pathway ccRCC was also found to have an altered metabolic phenotype. A TCGA research network study of this tumor type revealed frequently mutated genes involved in the Krebs cycle, decreased AMP-activated protein kinase (AMPK) levels (a central regulator of energy homeostasis), upregulation of glutamine transporter, and pentose phosphate pathway genes—all hallmarks of a Warburg-like effect.
Finally, chromophobe RCC also displays alterations in metabolic pathways. Alterations in mitochondrial DNA were found to be central to the pathogenesis of chromophobe RCC, implicating a unique mechanism of cancer-associated reprogramming of mitochondria.
These metabolic changes could offer alternative therapeutic opportunities. The potential for targeting the NRF2 pathway is being examined at the preclinical level. Meanwhile, it has been hypothesized that existing cancer therapies could be repurposed in rational combinations to target the metabolic phenotype.
Jane de Lartigue, PhD, is a freelance medical writer and editor based in New Haven, Connecticut.
Key Research
- Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26(3):319-330.
- Durinck S, Stawiski EW, Pavia-Jiménez A, et al. Spectrum of diverse genomic alterations define non—clear cell renal carcinoma subtypes. Nat Genet. 2015;47(1):13-21.
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branch evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883-892.
- Gerlinger M, Horswell S, Larkin J, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46(3):225-233.
- Gnjidić M, Fucak IK. Renal cell carcinoma: molecular pathways and targeted therapy. Period Biol. 2014;116(4):393-398.
- Kovac M, Navas C, Horswell S, et al. Recurrent chromosomal gains and heterogeneous driver mutations characterize papillary renal cancer evolution. Nat Commun. 2015;6:6336-6347.
- Srinivasan R, Ricketts CJ, Sourbier C, Linehan WM. New strategies in renal cell carcinoma: targeting the genetic and metabolic basis of disease. Clin Cancer Res. 2015;21(1):10-17.
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43-49.
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374(2):135-145.




