Role of Anti-PD-1/PD-L1 Immunotherapy in Cancer
Immunotherapy for the treatment of cancer has evolved alongside our improved understanding of the immune system.
Immunotherapy has become an increasingly appealing therapeutic strategy for patients with cancer, with many late-stage clinical trials demonstrating overall survival (OS) advantages in melanoma and castration-resistant prostate cancer. More recently, non-small cell lung cancer (NSCLC) has become a focus for the next generation of immune-based therapeutic strategies. Immunotherapy, in particular the use of monoclonal antibodies that block inhibitory immune checkpoint molecules and therefore enhance the immune response to tumors, has shown clinical promise in advanced solid tumors. The clinical rationale for targeting the programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) pathways will be reviewed in this supplement, including a comprehensive review of select ongoing clinical trials that evaluate the potential of targeted immunotherapy in cancer drug development. Emerging clinical data suggest that targeted immunotherapy in cancer will become an integral part of the clinical management strategy for solid tumors.
Introduction
Cancer is traditionally treated with conventional therapy (ie, chemotherapy or radiation therapy) or targeted drugs that directly kill tumor cells. While the number of patients who survive cancer has seen significant increases, the “war” rages on.1 More than a century ago, a series of primitive experiments hinted at the potential for harnessing the immune system to fight cancer.2 The immune system protects the body from foreign invading agents by recognizing “nonself” proteins (antigens) displayed on their surface that distinguish them from normal, healthy tissue (“self”). This subsequently initiates a protective response that neutralizes these organisms.3 William Coley was the first to draw a link between the immune system and cancer. He observed spontaneous remission in cancer patients following infection with a mixture of killed infectious agents, dubbed Coley’s toxins.2 In the years that followed, a dynamic and complex relationship between the immune system and cancer was uncovered, giving birth to the concept of immunotherapy.
Cancer cells are normal cells that have acquired numerous hallmark abilities that allow them to become malignant4; thus, they are essentially identified as “self”—part of the host. In spite of this, they often display unusual or inappropriate proteins on their cell surface that allow the immune system to identify them as “non-self,” and an antitumor immune response is often initiated. However, cancer cells have evolved a number of mechanisms to enable evasion of this immune response and render it ineffective. Typically, by the time a cancer becomes detectable, the balance of power between the immune system and the cancer has shifted in favor of the growing tumor, and a state of immune tolerance has been established. Immunotherapy comprises a diverse range of therapeutic approaches intended to harness the immune system to reestablish a targeted antitumor immune response. The goal of cancer immunotherapy is to enable the patient’s immune system to specifically recognize and kill cancer cells.5-9
There are 2 distinct types of immunotherapy: passive immunotherapy uses components of the immune system to direct targeted cytotoxic activity against cancer cells, without necessarily initiating an immune response in the patient, while active immunotherapy actively triggers an endogenous immune response. Passive strategies include the use of the monoclonal antibodies (mAbs) produced by B cells in response to a specific antigen.6 The development of hybridoma technology in the 1970s and the identification of tumorspecific antigens permitted the pharmaceutical development of mAbs that could specifically target tumor cells for destruction by the immune system. Thus far, mAbs have been the biggest success story for immunotherapy; the 3 best selling anticancer drugs in 2013 were mAbs.10 Among them is rituximab (Rituxan, Genentech), which binds to the CD20 protein that is highly expressed on the surface of B-cell malignancies such as non-Hodgkin’s lymphoma (NHL). Rituximab is approved by the US Food and Drug Administration (FDA) for the treatment of NHL and chronic lymphocytic leukemia in combination with chemotherapy.11 Another important mAb is trastuzumab (Herceptin; Genentech), which revolutionized the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer by targeting the expression of HER2.12
To actively drive an antitumor immune response, therapeutic cancer vaccines have been developed. Unlike the prophylactic vaccines that are used preventively to treat infectious diseases, therapeutic vaccines are designed to treat established cancer by stimulating an immune response against a specific tumor-associated antigen. In 2010, sipuleucel- T (Provenge; Dendreon Corporation) was approved by the FDA for the treatment of metastatic, castration-resistant prostate cancer based on results of the IMPACT (Immunotherapy Prostate Adenocarcinoma Treatment) trial in which it improved OS by 4.1 months and reduced the risk of death by 22% versus placebo.13,14 The advantage of active immunotherapies is that they have the potential to provide long-lasting anticancer activity by engaging both the innate and adaptive arms of the immune response. While mAbs are typically considered passive immunotherapies, there is increasing evidence that they also induce an adaptive immune response via a “vaccination-like” effect.15
Despite these successes, immunotherapy has previously faced skepticism and significant disappointment; however, it is now beginning to gather momentum, particularly following the discovery of immune checkpoints and the success of their therapeutic targeting.16 Growing appreciation of the ability of cancer cells to evade the immune response and understanding of how this impacts the development of cancer and resistance to cancer therapy has led researchers to investigate the mechanisms by which immune evasion occurs. This has resulted in recognition of the significant role that immune evasion plays in malignant progression.17
Generation of an effective antitumor immune response involves a series of steps that ultimately lead to the death of cancer cells (Figure 1).18 In the first step (step 1), cancerspecific antigens are released from cancer cells and captured by dendritic cells (a type of antigen-presenting cell [APC]). This step must be accompanied by immunogenic signals such as pro-inflammatory cytokines. Next, the dendritic cells present the captured antigen to the immune effector cells—cytotoxic T cells (step 2). This activates and primes the cytotoxic T cells to generate a specific immune response against the cancer-specific antigens (step 3). Activated T cells then traffic to (step 4) and infiltrate (step 5) the tumor and recognize cancer cells by their expression of the specific antigen. They then bind the specific antigen to their T-cell receptor (step 6). The cytotoxic T cell kills the cancer cell (step 7), which results in the release of additional cancer-specific antigens, thereby starting the whole process over again. This cycle ceases to function appropriately in patients with cancer, as tumors are able to break the cycle by affecting any of these 7 steps.18
One of the mechanisms by which cancer cells break this cycle is by hijacking immune checkpoint pathways that regulate T-cell responses (step 3) or their function (step 7). As such, significant research efforts have focused on the development of mAbs targeting these proteins. The checkpoint protein that has garnered the most attention is cytotoxic T-lymphocyte antigen-4 (CTLA-4). Ipilimumab, an antibody that targets CTLA-4, is approved by the FDA. Ipilimumab (Yervoy; Bristol-Myers Squibb) was approved in 2011 for the treatment of melanoma, representing the first new treatment option for melanoma in more than a decade, after demonstrating a clear survival advantage for patients.19 Clinical trials demonstrated that 46% of patients treated with ipilimumab were alive after 1 year, and 24% after 2 years.20
A number of other checkpoint proteins are also being examined. The PD-1 receptor and its ligands PD-L1 and PD-L2 are part of the same family of coregulatory molecules as CTLA-4. Several anti-PD-L1 agents are currently being investigated in both solid and hematologic cancers. In September 2014, pembrolizumab (Keytruda; Merck Sharp & Dohme Corp), previously known as MK-3475, was the first to be approved by the FDA. It is indicated for use in patients with advanced or unresectable melanoma who are no longer responding to other drugs.21 The safety profile of pembrolizumab in patients who did not achieve an adequate response to earlier ipilimumab treatment is similar to that observed in ipilimumab-naïve patients.22 Earlier in 2014, nivolumab (Bristol-Myers Squibb) was granted regulatory approval in Japan, also for unresectable melanoma.23 In this supplement, we focus on the clinical development of PD-1/ PD-L1-targeting agents.
Figure 1. The Generation of Antitumor Immunity18
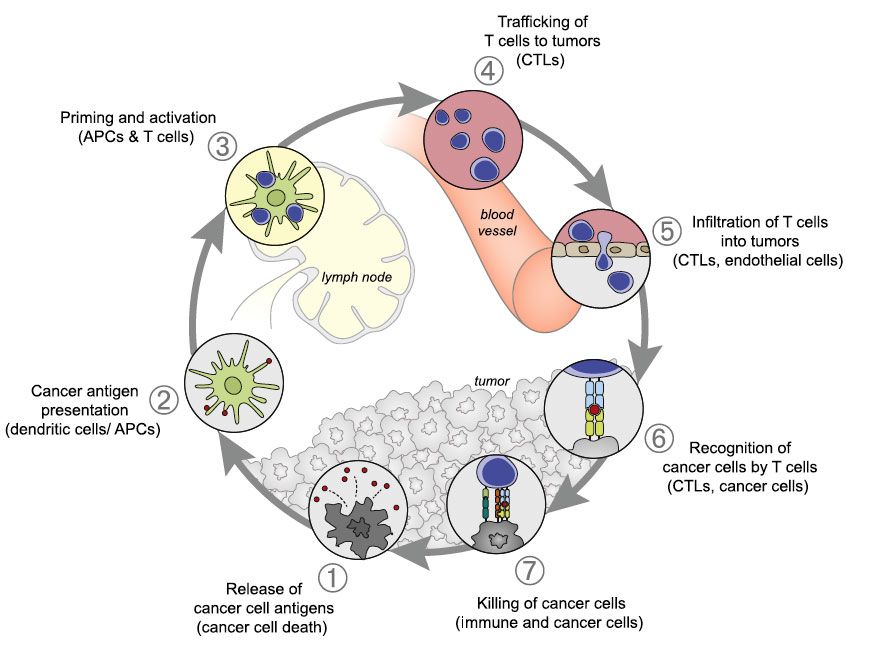
APC indicates antigen-presenting cell; CTL, cytotoxic T lymphocyte.
Generation of an effective antitumor immune response involves a series of stepwise events that ultimately form a cyclical response that increases the depth and breadth of the immune response against tumor-associated antigens. In cancer patients, this cycle functions suboptimally, allowing cancer cells to avoid death.
Reprinted with permission from Chen DS, Mellman I. Immunity. 2013;39:1-10.
The PD-1 and PD-L1 Pathway in Normal Human Physiology and Neoplasms
Activation of T cells during an immune response is a 2-step process: the first step gives the immune response specificity and requires interaction of T-cell receptors with a specific antigenic peptide-containing complex on APCs. This is followed by an antigen-independent coregulatory signal that determines if the T cell will be switched on or off. The secondary signal promotes T-cell clonal expansion, cytokine secretion, and functional activity of the T cell, and in the absence of this signal (even in the presence of a target antigen), T cells fail to respond effectively and are functionally inactivated. This is designed as a fail-safe mechanism to ensure that the immune system is activated at the appropriate time in order to limit collateral damage to normal tissue and minimize the possibility of chronic autoimmune inflammation. Checkpoint pathways regulate these coregulatory signals and can be either stimulatory (switching T cells on) or inhibitory (switching them off).8,24,25
The 2 known inhibitory checkpoint pathways involve signaling through the CTLA-4 and PD-1 receptors. These proteins are members of the CD28-B7 family of cosignaling molecules that play important roles throughout all stages of T-cell function. The PD-1 receptor (also known as CD279) is expressed on the surface of activated T cells. Its ligands, PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273), are expressed on the surface of APCs such as dendritic cells or macrophages. PD-L1 is the predominant ligand, while PDL2 has a much more restricted expression pattern. When the ligands bind to PD-1, an inhibitory signal is transmitted into the T cell, which reduces cytokine production and suppresses T-cell proliferation.5,8,26
PD-L1 has also been shown to bind to B7-1 (CD80), an interaction that also suppresses T-cell proliferation and cytokine production; however, the exact relative contributions of the PD-L1:PD-1 and PD-L1:B7-1 pathways in cancer remain unclear. The PD-1-targeting agents currently in development inhibit both pathways. However, as the binding sites for PD-1 and B7-1 are adjacent but not overlapping, agents that specifically target one or the other may be developed.27
CTLA-4 and PD-1 have distinct roles in regulating immunity (Figure 228), with both temporally and spatially distinct expression patterns. CTLA-4 regulates T-cell activity at initial activation and acts as a signal dampener, regulating the amplitude of early activation of naïve and memory T cells, while PD-1 functions to limit the activity of already activated T cells in the periphery during the inflammatory response to infection to limit autoimmunity.5,8,18,28,29
Figure 2. Targeting the Immune Checkpoints for Cancer Immunotherapy28
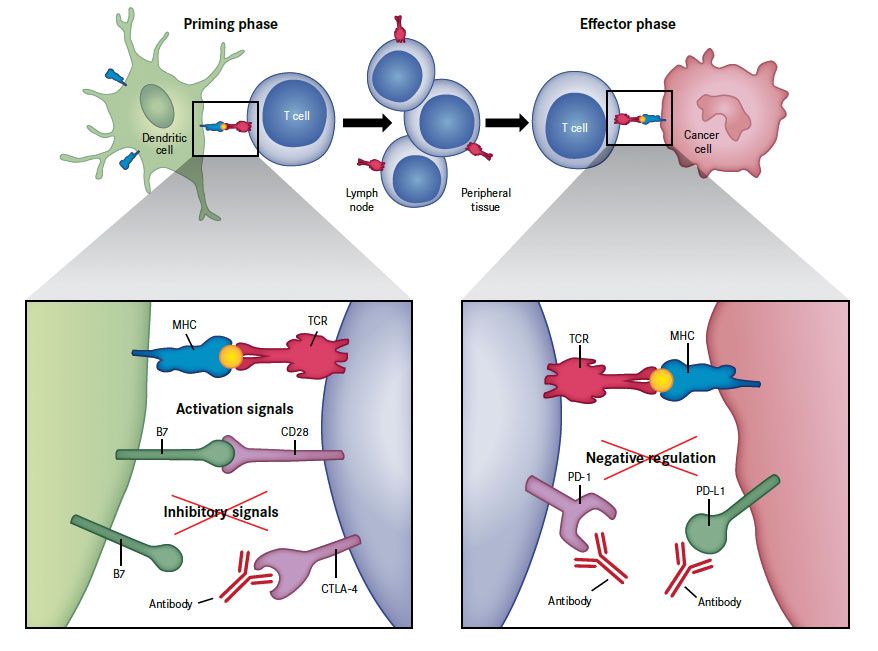
CD28 indicates cluster of differentiation 28; CTLA-4, cytotoxic T-lymphocyte antigen-4; MHC, major histocompatibility complex; PD-1, programmed death-1; PD-L1, programmed death ligand-1; TCR, T-cell receptor.
Activation of T cells is a 2-step process that requires recognition of specific antigens presented by MHC on the surface of cancer cells through their “primed” T-cell receptor, as well as a co-regulatory signal delivered by the B7 family of receptors (the so-called immune checkpoints). The 2 checkpoints that deliver inhibitor signals, CTLA-4 and PD-1, function at different points in T-cell function. CTLA-4 is upregulated shortly after activation and negatively regulates T-cell activation during the “priming” phase of T-cell response within the lymph nodes by binding to B7 molecules on the surface of antigen-presenting cells. Conversely, when these B7 molecules bind to CD28 instead, they generate the opposite, activating signals. PD-1 is expressed on T cells later on in the immune response, during the effector phase of T-cell response. When PD-1 binds to either of its ligands (PD-L1 or PD-L2), which are primarily expressed within inflamed tissues and the tumor microenvironment, it results in inhibition of T-cell activity. Blockade of CTLA-4 or PD-1/PD-L1 with antibodies results in the preferential activation of T cells with specificity for cancer cells.
From Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012; 366(26):2517-2519. Copyright © 2012 Massachusetts Medical Society. Reprinted with permission.
Cancer cells exploit the PD-1 pathway to create an immunosuppressive environment. There is often an increase in the production of inhibitory pathways and suppression of stimulatory pathways, allowing cancer cells to dampen down the immune response at inappropriate times to create an immunosuppressive environment in which they are able to thrive. Cancer cells drive high expression levels of PD-L1 on their surface, allowing activation of the inhibitory PD-1 receptor on any T cells that infiltrate the tumor microenvironment, effectively switching those cells off.5,8,27 Indeed, upregulation of PD-L1 expression levels has been demonstrated in many different cancer types (eg, melanoma [40%- 100%], NSCLC [35%-95%], and multiple myeloma [93%]), and high levels of PD-L1 expression have been linked to poor clinical outcomes.7,30-33 Furthermore, tumor-infiltrating T cells have been shown to express significantly higher levels of PD-1 than T cells that infiltrate normal tissue. It is thought that the tumor microenvironment may secrete pro-inflammatory cytokines, including interferon-gamma to upregulate the expression of PD-1 on tumor-infiltrating T cells to ensure that they can respond to the high levels of PD-L1 expressed on the tumor.34
Designing therapies that specifically target mechanisms of immune evasion is an attractive therapeutic approach because the ability of the tumor to suppress the immune response can seriously undermine the clinical efficacy of cancer therapies. Confirmation of the pivotal role of the PD-1 pathway in immunosuppression provided a strong rationale for the development of mAbs that block the PD-1 pathway, and several such agents are now being assessed in clinical trials.
Select Clinical Trials of Immunotherapy in Cancer
Pembrolizumab (MK-3475)
Pembrolizumab (Merck) is a humanized immunoglobulin G4 anti-PD-1 mAb. Pembrolizumab was evaluated in numerous phase l to phase lll trials (Table 1) in a variety of cancer types.35 There were 2 reports of interim results from a phase l study in patients with advanced, metastatic solid tumors (clinical trial identifier: NCT01295827). One report described the clinical safety and efficacy of pembrolizumab as monotherapy in 38 patients with previously treated NSCLC. Using immune-related response criteria (irRC), the objective response rate (ORR) was 24%, including squamous and non-squamous subtypes (most responses observed within 9 weeks of treatment initiation) and the median duration of response had not been reached. According to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria, the ORR was 21%. Pembrolizumab was generally well tolerated, with adverse events (AEs) observed in 21% (n = 8) of patients, most commonly fatigue, rash, and pruritus (16% each). Only 1 case of a grade 3/4 drug-related AE was reported (pulmonary edema).36
Table 1. Phase II and III National Clinical Trials Targeting PD-1/PD-L1 in Cancer35
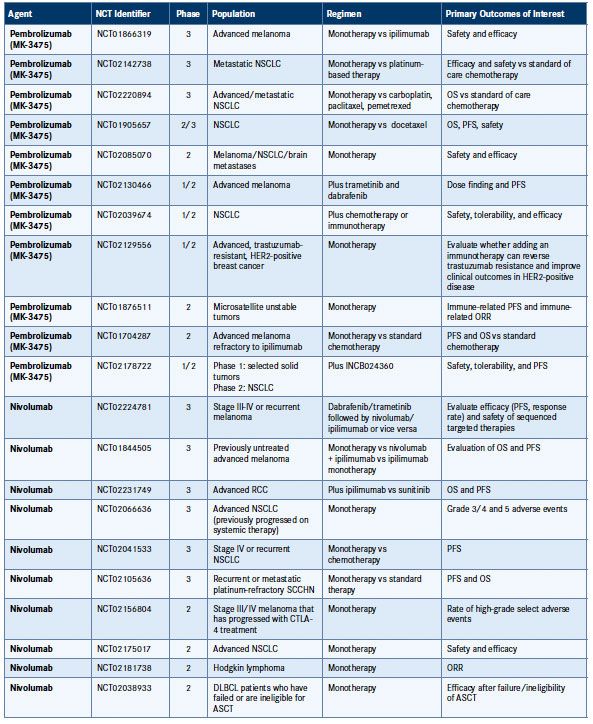
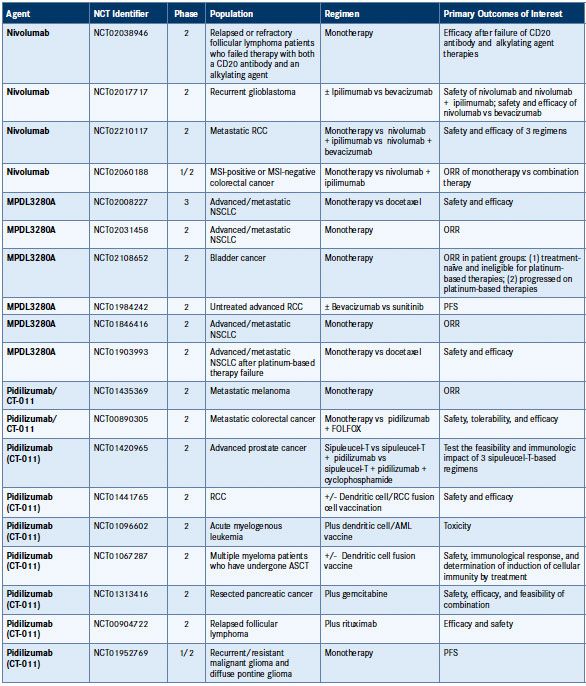
AML indicates acute myeloid leukemia; ASCT, autologous stem cell transplant; CTLA-4, cytotoxic T-lymphocyte antigen-4; DLBCL, diffuse large B-cell lymphoma; FOLFOX, folinic acid + fluorouracil + oxaliplatin; HER2, human epidermal growth factor receptor 2; MSI, microsatellite instability; NCT, national clinical trial; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck.
The second report involved an expansion study of a cohort of 294 patients with melanoma and with (n = 179) or without (n = 115) previous ipilimumab treatment. Pembrolizumab was administered intravenously as monotherapy at a dose of 2 mg/kg or 10 mg/kg every 2 or 3 weeks until disease progression or unacceptable toxicity was observed. ORRs per RECIST 1.1 and irRC were very similar and were greater than 35% across all doses and schedules, including both ipilimumab-naïve and ipilimumab-pretreated patients. Median duration of response had not yet been reached, while median progression-free survival (PFS) exceeded 8 months. Pembrolizumab was also well tolerated in this study, with manageable toxicity in patients with melanoma; grade 3/4 drug-related AEs were reported in 10% of patients, and included hypothyroidism and hyperthyroidism.37
The KEYNOTE-001 program involves a very large, multipart phase l study designed to evaluate the safety and efficacy of pembrolizumab, at several doses, in patients with advanced melanoma and NSCLC.35 Data from the 1.5-year follow-up analysis to the initial dose-expansion cohort for melanoma were reported by Dr Richard Kefford at the 2014 American Society of Clinical Oncology (ASCO) Annual Meeting. This nonrandomized cohort of 135 patients received pembrolizumab at a dose of 10 mg/kg every 2 weeks or 10 mg/kg every 3 weeks. Ipilimumab-naïve (IPI-N) patients could also receive a dose 2 mg/kg every 3 weeks.
BRAF mutations were less common than expected (22%), lactic acid dehydrogenase was elevated in 31% of patients, and 58% of patients had stage M1c disease. Clinical activity was observed in both IPI-N and ipilimumab-refractory (IPI-R) patients. The overall complete response rate was 9% (IPI-N, 12%; IPI-R, 5%) and the ORR was 41% (IPI-N, 43%; IPI-R, 38%); 88% of responses were ongoing at the time of analysis, with median duration of response not reached. Overall median PFS was 7 months; the 6-month PFS rate was 51%. Median OS was not reached at any dose. PD-L1 staining by immunohistochemistry was observed in 71% of evaluable tumors (n = 125). PD-L1 expression correlated with response. ORR was 49% for PD-L1-positive patients versus 13% for PD-L1-negative patients.22,38
Another analysis of the KEYNOTE-001 melanoma cohort compared patients who were IPI-N with those who were IPIR. Patients were randomized to receive pembrolizumab 2 mg/kg every 3 weeks or 10 mg/kg every 3 weeks. More than half of patients had stage M1c disease. Approximately half of the IPI-N patients received no prior treatment, whereas the majority of IPI-R patients received multiple lines of therapy. Median follow-up was 12 months in the IPI-N group (n = 103) and 8 months in the IPI-R group (n = 173). ORR and median PFS were similar between the 2-mg/kg and 10-mg/ kg doses. ORRs using RECIST 1.1 criteria by central review for the IPI-N patients receiving 2 mg/kg or 10 mg/kg were 33% and 40%, respectively. ORRs in IPI-R patients were 26% for each dose. Investigator review of irRC was comparable to central review of ORR and median duration of response was not reached. PFS rates at 12 months for the IPI-N group treated with the 2-mg/kg and 10-mg/kg doses of pembrolizumab were 50% and 48%, respectively. For IPI-R patients, 12-month PFS rates were 44% and 37%, respectively. The OS rate at 12 months was 68% in IPI-N patients and 60% in IPI-R patients. The maximum percent baseline change in tumor size was 71% for both IPI-N and IPI-R patients.22
Although almost all patients reported AEs and approximately one-third were serious AEs, toxicity-related discontinuation of therapy was minimal, with most discontinuations occurring in the IPI-R group. Immune-mediated AEs were uncommon except for hypothyroidism, which was low grade and reported in 32 patients. Rash was reported in 18% of IPI-N patients and 18% of IPI-R patients. Grade 3/4 rash was rare. Overall, the results suggest that pembrolizumab is a promising treatment for advanced melanoma, with a recommended dosage of 2 mg/kg every 3 weeks.39
Results from the IPI-R cohort were published in The Lancet (Robert et al, 2014). Importantly, pembrolizumab at both doses evaluated was safe and effective in patients with advanced melanoma that progressed after treatment with ipilimumab, a BRAF or MEK inhibitor, or both. These findings are highly promising considering the lack of therapy options in this setting.22
A pooled analysis of the 3 melanoma cohorts in KEYNOTE- 001 was reported by Dr Antoni Ribas at the 2014 ASCO Annual Meeting. The study included 411 patients and evaluated several pembrolizumab doses (10 mg/kg every 2 weeks, 10 mg/kg every 3 weeks, and 2 mg/kg every 3 weeks). The ORR was 34%, with 88% of responses ongoing at the time of data lock. The ORR in IPI-N (ie, treatment-naïve) and ipilimumab-treated patients was 40% and 28%, respectively. Median PFS was 5.5 months. Median OS was not reached; the 12-month and 18-month OS rates were 69% and 62%, respectively. Clinical activity was observed at all doses evaluated. Serious treatment-related AEs were seen in 8% of patients; however, only 4% discontinued treatment because of drug-related toxicities. The most common toxicities of any grade were fatigue, pruritus, and rash.40 Lung cancer data from the KEYNOTE-001 study was also reported at the 2014 Annual Meeting by Dr Naiyer Rizvi. Pembrolizumab was evaluated as first-line therapy for locally advanced or metastatic NSCLC. Patients were randomized to pembrolizumab 10 mg/kg every 2 weeks or every 3 weeks. The first 11 patients were randomized to a 2-mg/kg or 10-mg/kg dose. Of 84 patients, 57 had confirmed PD-L1-expressing tumors. The ORR was 26% by RECIST 1.1 criteria and 47% by irRC. Median PFS was 27 weeks by RECIST and 37 weeks by irRC. Almost all responses were maintained at the time of analysis. More than half of patients had treatment-related toxicities. However, most were grade 1/2. There were 2 grade 3 adverse events reported, pericardial effusion and pneumonitis, and 1 grade 4 adverse event, an increase in blood creatinine phosphokinase. These data suggest that pembrolizumab is well tolerated and clinically active as initial treatment for advanced NSCLC. Upcoming comparative studies planned for pembrolizumab in NSCLC include 1 study with platinum- based chemotherapy (KEYNOTE-024) and 1 study with docetaxel (KEYNOTE-010).41
Nivolumab (BMS-936558)
The first agent targeting the PD-1 pathway to enter clinical testing was BMS-936558 (nivolumab/ONO-4538, Bristol- Myers Squibb; formerly MDX-1106). It is a fully human immunoglobulin G4 mAb that targets PD-1. Nivolumab was first evaluated in a phase l multicenter trial involving small cohorts of 6 patients with advanced, treatment-refractory solid tumors treated with single doses of 0.3, 1.0, 3.0, or 10 mg/kg nivolumab, followed by an expansion cohort of 15 patients who received 10 mg/kg nivolumab. Nivolumab induced a durable complete response (CR) in 1 patient with colorectal cancer at a dose of 3 mg/kg and partial responses in 1 patient with melanoma and renal cell carcinoma (RCC) at a dose of 10 mg/kg (clinical trial identifier: NCT00441337).42
A total of 304 heavily pretreated patients with advanced solid tumors have been enrolled since 2008, including those with NSCLC (n = 129), melanoma (n = 107), and RCC (n = 34). Patients received 0.1 to 10 mg/kg intravenous nivolumab every 2 weeks, and tumors were assessed by RECIST 1.0 criteria after each 4-dose cycle, up to a maximum of 12 doses or until unacceptable toxicity, confirmed progression, or a CR occurred. Durable objective responses were observed (Table 236,38,40,43-63), with 28 of 54 responders having an objective response lasting 1 year or longer. A sustained OS benefit was observed across tumor types, with 61% and 44% (melanoma); 43% and 32% (NSCLC); and 70% and 52% (RCC) of patients alive at 1 and 2 years, respectively (see Table 2 for median OS).44 In a separate assessment of patients with NSCLC, an OS benefit was also observed across histologies, with 39% and 43% of patients with squamous and non-squamous NSCLC alive after 1 year, respectively.45 AEs of any grade occurred in 41% (n = 53) of patients, while grade 3/4 AEs occurred in 5% (n = 6).45
Updated results in the melanoma cohort were presented at the 2014 ASCO Annual Meeting. A total of 32% of the 107 patients experienced an objective response to nivolumab therapy (as determined by RECIST criteria) in all dose groups. The dose that will be used for the phase lll trial is 3 mg/kg, and in that cohort, the ORR was 41%. Overall, median PFS and OS were 3.7 months and 17.3 months, respectively.
In the 3 mg/kg group, median PFS and OS were 9.7 months and 20.3 months. Responses were enduring; median duration of response was 22.9 months. The OS rate at 1, 2, and 3 years was 63%, 48%, and 41%, respectively. Immune-related responses were similar to those assessed by RECIST criteria.46
Results of an open-label, randomized phase lll study (Checkmate-037, clinical trial identifier: NCT01844505) were reported by Dr Jeffrey Weber at the European Society for Medical Oncology 2014 Congress. In this study, 370 patients with advanced melanoma that progressed after anti- CTLA-4 and BRAF-targeted therapy were randomized 2:1 to nivolumab 3 mg/kg every 2 weeks or investigator’s choice chemotherapy (ICC). ORR by central review, reported after at least 6 months of follow-up in the first 120 nivolumabtreated patients and 47 chemotherapy-treated patients, was 32% and 11%, respectively. Duration of response was not reached for nivolumab-treated patients, with most responses ongoing, compared with 3.6 months among patients receiving chemotherapy. Grade 3/4 adverse events occurred in 9% of patients in the nivolumab group and in 31% of those in the ICC group. Nivolumab was well tolerated and was associated with durable responses, confirming the potential for immunotherapy in patients with advanced melanoma.64
A randomized phase ll trial of nivolumab monotherapy showed promising efficacy in 168 patients with clear-cell metastatic renal cell carcinoma (mRCC). Patients were stratified by Memorial Sloan Kettering Cancer Center risk score and prior lines of therapy for metastatic disease and then randomized to nivolumab 0.3 mg/kg, 2 mg/kg, or 10 mg/kg.
No dose relationship was observed for PFS. Median PFS was 2.7, 4.0, and 4.2 months, respectively. Median OS was 18.2, 25.5, and 24.7 months, respectively, comparing favorably to studies of tyrosine kinase therapy in mRCC. Median OS in favorable-risk patients was not reached; median OS in intermediate- and poor-risk patients was 20.3 weeks and 12.5 weeks, respectively. For patients who received 1 line of prior therapy, OS was not reached, and for patients who received more than 2 lines of prior therapy, median OS was 18.7 months. Further research of nivolumab in mRCC includes 2 phase lll trials, 1 in previously treated patients comparing survival with everolimus and 1 in combination with ipilimumab in the first-line setting.51 In addition to being studied as monotherapy in patents with mRCC, nivolumab was evaluated in a phase I study in combination with sunitinib or pazopanib. Patients were randomized to treatment with sunitinib 50 mg or pazopanib 800 mg plus nivolumab 2 mg/kg every 3 weeks, with planned escalation to nivolumab 5 mg/kg. Almost half of the patients in the sunitinib group (n = 33) and all patients in the pazopanib group (n = 20) received previous therapy. The ORR was 52% for the sunitinib group and 45% for the pazopanib group. Median PFS was 48.9 and 31.4 weeks, respectively. Responses were evident at the 6-week assessment in 41.2% of sunitinib-treated patients and 55.6% of pazopanib-treated patients; 58.8% and 33.3% of responses, respectively, were ongoing at the time of analysis. The preliminary results appear promising, with efficacy better for combination therapy with nivolumab than for either agent alone. However, investigators are cautiously optimistic due to higher than expected liver and kidney toxicity.52
PD-L1 expression is a poor prognostic factor in patients with ovarian cancer. Thus, PD-1/PD-L1 blockage serves as a rationale therapeutic target for this disease. At the 2014 ASCO Annual Meeting, Dr Junzo Hamanishi presented encouraging data for nivolumab in patients with advanced or relapsed, platinum-resistant ovarian cancer. Nivolumab was administered every 2 weeks at a dose of 1 mg/kg or 3 mg/kg for up to 4 cycles. The ORR was 23% in 13 treated patients, including 2 partial responses at the 1 mg/kg dose and 1 partial response at the 3-mg/kg dose.55
Nivolumab is also being evaluated in a phase l trial in combination with the CTLA-4-targeting agent ipilimumab (clinical trial identifier: NCT01024231). The rationale for this study is that targeting a single inhibitory T-cell pathway may not be sufficient to reestablish correct T-cell functioning and that synergistic activity may be found by inhibiting both pathways simultaneously. Patients with advanced melanoma (n = 53) were treated with escalating doses of concurrent therapy with nivolumab and ipilimumab every 3 weeks for 4 doses, followed by nivolumab alone every 3 weeks for 4 doses. Combined treatment was then administered every 12 weeks for up to 8 doses. A sequenced regimen (n = 33) was also examined, in which patients previously treated with ipilimumab received nivolumab every 2 weeks for up to 48 doses. The combination of nivolumab and ipilimumab induced clinical activity (according to modified World Health Organization [WHO] criteria) that appeared to be distinct from monotherapy with either agent, with rapid and deep tumor regression observed in many patients.
ORRs were 40% and 20% in the concurrent-regimen and sequenced-regimen groups, respectively. In the concurrent regimen group, 53% of patients had an objective response, with all having tumor reduction of at least 80%. Clinical activity (conventional, unconfirmed, or immune-related response or stable disease for ≥24 weeks) was observed in 65% of patients.65 In updated results reported at the 2014 ASCO Annual Meeting, 1- and 2-year OS rates in the concurrent therapy cohort were impressive, at 85% and 79%, respectively. Median ORR was 42%. Median PFS and OS were 27 weeks and 40 months, respectively. In the cohort of 17 patients receiving nivolumab 1 mg/kg and ipilimumab 3 mg/kg, 1- and 2-year OS rates were even higher, at 94% and 88%, respectively. No new safety signals were reported. Neither BRAF mutation status nor PD-L1 expression appeared to impact clinical activity. Phase 2/3 studies of combination therapy are ongoing.49,66
Early results from another phase l study reported at the 2014 ASCO Annual Meeting looked at the combination of nivolumab and ipilimumab in 43 patients with either previously treated or untreated mRCC (clinical trial identifier: NCT01472081). Patients were randomized to treatment with nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (N3 + I1) or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (N1 + I3) every 3 weeks for 4 doses. Both groups then received nivolumab 3 mg/kg every 2 weeks until progression or toxicity. The ORR was 29% for the N3 + I1 group and 39% for N1 + I3 group. Median PFS was 28.1 weeks and 26.1 weeks, respectively. A phase lll trial is planned in the first-line setting of mRCC based on the encouraging clinical activity and manageable safety profile reported from this study.50
Two other phase l combination trials are currently under way with nivolumab (clinical trial identifiers: NCT01454102 and NCT01176461). In the first, nivolumab is combined with platinum-based chemotherapy (gemcitabine/cisplatin, pemetrexed/cisplatin, or carboplatin/paclitaxel) in patients with chemotherapy-naïve advanced NSCLC. A total of 43 patients were treated with escalating doses of nivolumab, starting at 10 mg/kg every 3 weeks until progression and chemotherapy doublets for 4 cycles at standard dosing. According to RECIST 1.1 criteria, total ORRs were 43% (gemcitabine/ cisplatin), 40% (pemetrexed/cisplatin), and 31% (carboplatin/paclitaxel).48 In the second trial, patients naïve to ipilimumab or who failed prior ipilimumab therapy were treated with nivolumab at 1.0, 3.0, or 10 mg/kg in combination with a peptide vaccine. Response rates by RECIST criteria were 28% in ipilimumab-naïve patients (n = 34) and 32% in patients who failed ipilimumab therapy (n = 46).67 A key question in the first clinical trials of PD-1 pathway agents was whether they had significant potential for inducing autoimmune AEs given previous clinical experience with ipilimumab, which induces moderate-to-severe autoimmune-type AEs, including hepatitis, endocrinopathies, and dermatitis. However, PD-1 agents have been generally well tolerated and induce only a low rate of autoimmune-type AEs that are usually manageable with the use of immunosuppressants.
In the phase l trial of patients with advanced solid tumors, drug-related AEs of any grade occurred in 72% of patients, while grade 3/4 AEs occurred in 15%.44,45 Combination therapy with ipilimumab was also associated with an acceptable level of AEs at the maximum doses. Drug-related grade 3/4 AEs occurred in 53% of patients treated with concurrent therapy and in 18% of those treated with sequential therapy, with the most common AEs being rash, pruritus, fatigue, and diarrhea in the concurrent group and elevated lipase levels in the sequential group.65 Likewise, combination with platinum-based chemotherapy was well tolerated; 49% of patients experienced drug-related grade 3/4 AEs, including pneumonitis, rash, and colitis, which were manageable. The study in patients who had previously failed ipilimumab therapy indicated that nivolumab did not induce the same immune-related AEs as ipilimumab.48,67
Several phase ll and lll clinical trials of nivolumab in patients with NSCLC and melanoma have also recently been initiated; results are not yet available (Table 135).
Pidilizumab (CT-011)
Pidilizumab (CT-011; CureTech) is a humanized anti-PD-1 immunoglobulin G1-kappa mAb. Positive phase 1 clinical trials showed that a single dose of pidilizumab (0.2 to 0.6 mg/kg) was generally safe and well tolerated, and preliminary clinical activity was observed, including 1 complete remission in a patient with follicular lymphoma (FL), 4 cases of stable disease (various hematologic malignancies), and 1 minimal response in a patient with acute myeloid leukemia.68
An international phase ll program was subsequently initiated to explore the safety and efficacy of pidilizumab in hematologic malignancies and solid tumors. A number of studies are ongoing (Table 135), while 1 study in patients with relapsed FL was recently completed. Results from this study were presented at the 2012 American Society of Hematology Annual Meeting. Thirty patients with rituximab-sensitive relapsed FL were treated with 3 mg/kg intravenous pidilizumab every 4 weeks for 4 infusions in combination with rituximab dosed at 375 mg/m2 weekly for 4 weeks, starting 2 weeks after the first infusion of pidilizumab.
Table 2. Clinical Efficacy Data for Agents Targeting PD-1/PD-L136,38,40,43-63
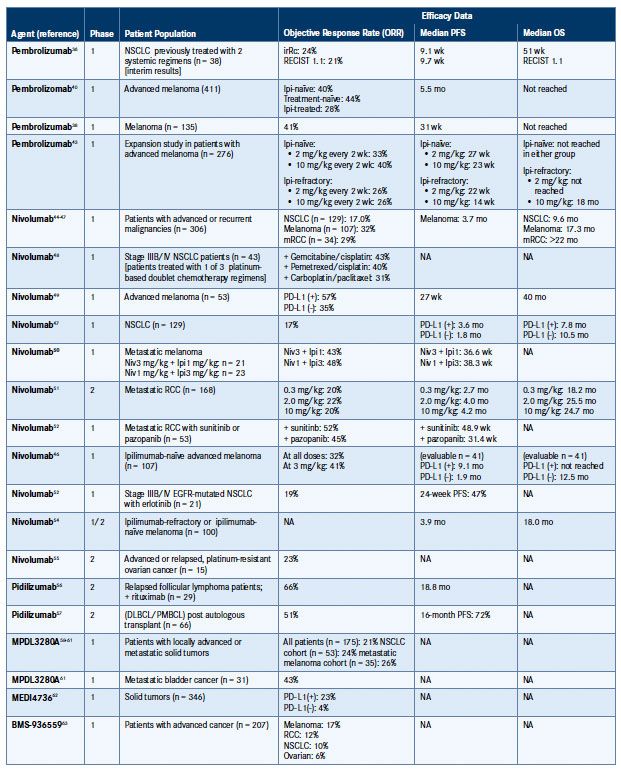
DLBCL indicates diffuse large B-cell lymphoma; EGFR, epidermal growth factor receptor; Ipi, ipilimumab; irRc, immune-related response criteria; mRCC, metastatic renal cell carcinoma; NA, not available/reported; Niv, nivolumab; NSCLC, non-small cell lung cancer; OS, overall survival; PD-L1, programmed death ligand-1; PFS, progressionfree survival; PMBCL, primary mediastinal large B-cell lymphoma; RCC, renal cell carcinoma; RECIST, Response Evaluation Criteria In Solid Tumors.
An ORR of 66% was achieved; a CR was observed in 52% of patients and a partial response in 14%, with measurable tumor regression in 86% of patients. Median time to response was 88 days, reflecting the delayed action of immunotherapies; indeed, 21% of patients achieved an initial response more than 4 months after first treatment. Median PFS was 18.8 months and was not reached for responders. The combination of pidilizumab and rituximab in this population was well tolerated, and no grade 3/4 drug-related AEs were observed.56
In another phase ll study, Armand et al evaluated the use of pidilizumab in patients with diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma after autologous hematopoietic stem-cell transplantation. Results in 66 patients showed 34% to be in complete remission, with an ORR of 51% among patients with measurable disease after transplantation. The study met its prespecified primary end point, with a 16-month PFS of 72%. The 16-month OS for eligible patients was 85%. The outcomes of this study demonstrate safety and clinical activity of PD-1 blockade in patients with DLBCL.57
Pidilizumab was also evaluated in the setting of metastatic melanoma in an open-label phase ll trial of heavily pretreated patients with stage IV progressive disease. The study randomized 103 patients to pidilizumab 1.5 mg/kg or 6.0 mg/kg; half of the patients in each group had received prior ipilimumab therapy. The ORR was 6%. Despite the low response rate, 12-month OS was 64.5%, which is comparable to results from studies of other PD-1 inhibitors in this setting. No significant difference in survival was seen based on exposure to ipilimumab. Pidilizumab was well tolerated; 4% of patients experienced serious AEs related to the study drug, including appendicitis, arthritis, hepatitis, and pneumonitis. Although initial results look promising for pidilizumab, the survival results of this study need confirmation in a randomized clinical trial.69
MEDI4736
MEDI4736 is a uniquely engineered human immunoglobulin G1-kappa monoclonal antibody targeting the PD-L1/ PD-1 pathway. It contains a triple mutation in the fragment crystallizable (Fc) domain to remove antibody-dependent cellular cytotoxicity (ADCC) activity and has high affinity and selectivity for PD-L1. MEDI4736 is currently being investigated in multiple advanced solid tumors as part of the nonrandomized phase 1/2 MEDI4736-1108 doseescalation and expansion study (clinical trial identifier: NCT01693562).35,70 Early results in 346 patients were reported at the 2014 ASCO Annual Meeting by Dr Neil Segal.
In the safety analysis, AEs were reported in 39% of patients, 6% of which were grade 3/4; 2% were serious AEs, with only 1 event leading to discontinuation of therapy. The most common events of any grade were fatigue (13%) and rash/pruritus (9%). Most notable were 3 cases of grade 3 aspartate transaminase (AST)/alanine transaminase (ALT) elevation.
Early and durable responses were attained, typical of those seen with other PD-1/PD-L1-targeted agents. Likewise, responses were correlated with PD-L1 expression status. For all patients treated with the 10-mg/kg dose, the ORR was 23% for PD-L1-positive patients and 4% in PD-L1-negative patients. In the NSCLC cancer cohort, ORRs were 39% and 5% for PD-L1-positive and -negative patients, respectively.
For patients in the head and neck squamous cell carcinoma (HNSCC) group, ORRs were 50% and 6%, respectively. To date, clinical activity with MEDI4736 has been observed in patients with NSCLC, HNSCC, pancreatic cancer, and gas troesophageal cancers.62 The randomized phase lll PACIFIC study will evaluate survival with MEDI4736 following concurrent chemoradiation in patients with stage III unresectable NSCLC (clinical trial identifier: NCT02125461). MEDI4736 is also being evaluated in combination with tremelimumab in patients with NSCLC.35,71
BMS-936559
BMS-936559 (Bristol-Myers Squibb) is a fully human immunoglobulin G4 anti-PD-L1 mAb that inhibits the binding of the PD-L1 ligand to both PD-1 and CD80. The results of a phase l clinical trial of BMS-936559 in patients with advanced cancer were reported at the 2012 ASCO Annual Meeting and were published in The New England Journal of Medicine later that year. A total of 207 patients including those with NSCLC (n = 75), melanoma (n = 55), RCC (n = 17), and ovarian cancer (n = 17) were treated with escalating doses of BMS-936559 (0.3, 1.0, 3.0, and 10 mg/kg). ORRs of 6% to 17% were observed depending on cancer type across all doses (Table 2).63
For patients with melanoma, the most significant objective response was observed at a dose of 3 mg/kg (29%), while for other cancer types it was observed with the 10- mg/kg dose. For patients with NSCLC, similar response rates were seen for squamous and non-squamous histologies (8% and 11%, respectively) across all doses. The response in NSCLC was unexpected because NSCLC has been considered to be non-immunogenic and poorly responsive to immunotherapy. Observed responses were durable across the multiple tumor types, and lasted for at least 1 year in half of the patients, with at least 1 year of followup.
Brahmer and colleagues found this particularly notable given the advanced stage of disease and number of previous treatments administered to patients. BMS-936559 was well tolerated, with grade 3/4 drug-related toxicities observed in only 9% of patients.63
MPDL3280A
MPDL3280A (Roche) is a human anti-PD-L1 mAb that contains an engineered Fc domain designed to optimize efficacy and safety by minimizing ADCC.58 The theory is that this structure will allow inhibition of the PD-1/PD-L1 interaction while minimizing the ADCC-mediated depletion of activated T cells that is required for an effective antitumor immune response.5
MPDL3280A has been evaluated in a phase l trial in patients with locally advanced or metastatic solid tumors. A total of 175 patients have been recruited to date. MPDL3280A was administered as a single agent at escalating doses of 1.0 or less, 3.0, 10, 15, and 20 mg/kg for a median duration of 127 days.58 The results of 2 expansion cohorts have also been reported; a cohort of 85 patients (53 of whom were evaluable for efficacy) with squamous or nonsquamous NSCLC and a cohort of 45 patients with metastatic melanoma (35 of whom were evaluable for efficacy).
In both cohorts, doses of 1.0 or less, 10, 15, and 20 mg/kg MPDL3280A were administered every 3 weeks for up to 1 year. MPDL3280A demonstrated durable responses and was well tolerated; efficacy data are summarized in Table 2.59,60 Of the 85 patients in the NSCLC cohort, 55% were heavily pretreated with at least 3 prior therapies, and 81% were smokers or ex-smokers. The 24-week PFS rate was 44% in squamous cell NSCLC and 46% in non-squamous cell NSCLC.59
All 175 patients in the initial trial and all patients in the NSCLC and melanoma expansion cohorts were evaluated for safety; the incidence of grade 3/4 drug-related AEs was 39%, 34%, and 34%, respectively. In the NSCLC cohort, AEs included pericardial effusion, dehydration, and dyspnea, while in the melanoma cohort, they included hyperglycemia and elevations in ALT and AST levels. No grade 3/4 pneumonitis was reported in any patients.58-60
Very encouraging results in 67 heavily pretreated patients with bladder cancer enrolled in the phase 1a expansion study were reported by Dr Thomas Powles at the 2014 ASCO Annual Meeting. Metastatic bladder cancer is an area of unmet need and is associated with a poor prognosis. After only 6 weeks of follow-up, MPDL3280A given at a dose of 15 mg/kg intravenously every 3 weeks for up to 16 cycles was associated with an ORR of 43% in patients with PD-L1- positive tumors. After 12 weeks of follow-up, the ORR was 52%. In patients with PD-L1-negative tumors, the ORR was 11%. Responses were rapid and enduring, with a median time to response of 42 days. Sixteen of 17 responses were ongoing at data cutoff, including 2 CRs. MPDL3280A was well tolerated, even in patients with renal insufficiency. Grade 3/4 AEs were rare (4%). Importantly, no renal toxicity was reported. Treatment with MPDL3280A was associated with transient increases in circulating CD8+Ki-67+ T cells and plasma proteins upstream of interferon gamma signaling, correlating to pharmacodynamic biomarkers of activity. MPDL3280A has now received breakthrough status from the FDA and research in bladder cancer is ongoing.61
Potential Role of PD-L1 as a Biomarker
Research and ongoing clinical studies are being conducted to evaluate the potential significance of PD-L1 as a biomarker for cancer immunotherapy.59 PD-L1-positive cancers are associated with poorer prognoses than those that are PD-1 negative. A correlation of PD-L1 expression and ORR was demonstrated in patients with the highest levels of PD-L1 expression (IHC 3; defined as ≥10% PD-L1- positive tumor-infiltrating immune cells) at 83% (5 of 6 patients; 95% CI, 40.2-99.1).59 Overall, PD-L1 is expressed in tumors and is thought to function as a key component of the cancer-immunity cycle by preventing the immune system from destroying cancer cells. In a phase l biomarker study, Th1-driven CD8 biology, intratumoral characteristics, and adaptive PD-L1 enhancement with MPDL3280A correlated with observed clinical responses and PD-L1 status.72 The potential role of PD-L1 as a biomarker remains to be elucidated.
Defining Immune-Related Response Criteria
In the late 1970s, the WHO developed response criteria to standardize the assessment of responses to cytotoxic anticancer agents in clinical trials and to facilitate the comparison of data between trials.73 This was followed by the development of RECIST criteria at the turn of the millennium to provide more simplified and standardized response definitions. 74 Although they have been updated and modified throughout the years,75 researchers have relied on these response criteria for decades when assessing the impact of novel agents in the treatment of cancer—from conventional chemotherapies to targeted therapies.
These criteria assume that an increase in tumor growth and/or the appearance of new cancerous lesions early in the course of treatment indicates progression, and it was recommended that treatment be stopped once this was observed. Thus, the term progression became synonymous with drug failure. However, with the development of immunotherapies that have a mechanism of action very different from that of traditional cytotoxic anticancer agents, many clinicians began to note different patterns of response to these drugs that were not adequately described by the existing criteria.
A series of initiatives in the first decade of the 2000s brought together experts in oncology, immunotherapy, and regulatory areas to determine whether novel response criteria could be developed that would more accurately reflect the results of immunotherapy treatment. Their main conclusions were that clinical activity often appears to be delayed following immunotherapeutic treatment and a period of apparent progression (as defined by the existing response criteria) may occur, followed by a response. Thus, discontinuation of immunotherapy at the point of apparent progressive disease may not be an appropriate course of action.76,77
Based on these conclusions, a series of large multinational studies were conducted using the most comprehensive data set available for immunotherapy: the phase ll clinical program with ipilimumab, involving 3 studies totaling 487 patients with advanced melanoma.72-74 The group noted 4 distinct response patterns, 2 conventional ones (immediate response and durable stable disease), and 2 that were unique to immunotherapy (response after tumor burden increase and response in the presence of new lesions). These unique responses probably reflect the dynamics of the immune system, which is engaged by immunotherapeutic agents. Rather than direct cytotoxic activity on tumor cells, immunotherapies have a more delayed mechanism of action, driving the expansion of T cells, which then infiltrate the tumor and kill tumor cells. Thus, the early increase in tumor burden that is often observed may be a result of the infiltration of T cells into the tumor. To more effectively capture these novel responses, the irRC were developed.
A comparison of the conventional response criteria and the irRC is presented in Table 3.77,78 Essentially, the irRC are based on modified WHO criteria and involve the use of bidimensional measurements on radiographic assessments of cancerous lesions (the longest diameter and the longest perpendicular diameter), as opposed to the unidimensional measurements employed by RECIST. Importantly, the irRC assess tumor burden differently: tumor burden is considered a continuous variable and the irRC incorporate measurements of both preexisting lesions (index lesions) and new lesions, as opposed to conventional criteria, which only consider index lesions. Thus, while new lesions always define progressive disease according to RECIST/WHO criteria, according to the irRC, in the absence of rapid clinical deterioration, they merely preclude a CR until progression is confirmed.76,77
Table 3. Immune-Related Response Criteria (irRC)77,78
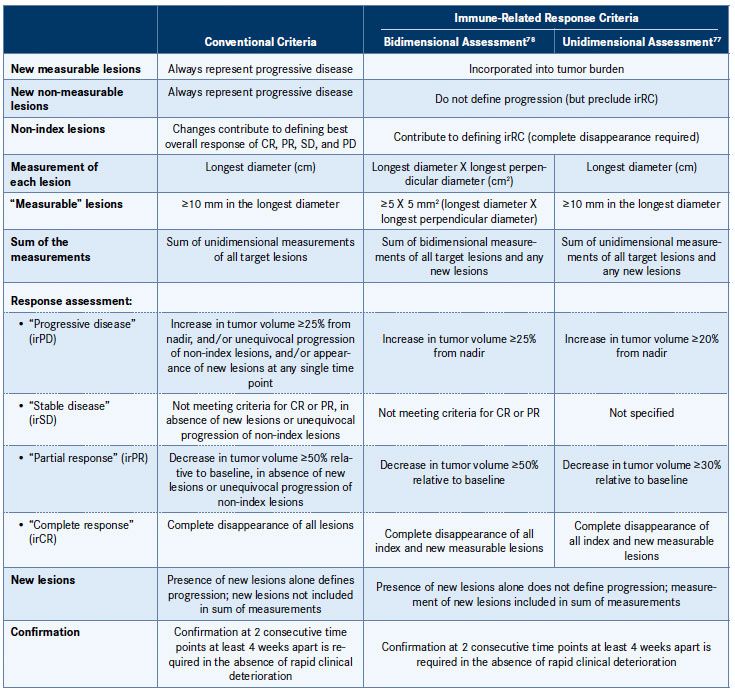
CR indicates complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Adapted from Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Clin Cancer Res. 2013;19(14):3936-3943 and Wolchok JD, Hoos A, O’Day S, et al. Clin Cancer Res. 2009;15(23):7412-7420.
The irRC are considered clinically meaningful, as they appear to be related to favorable survival; however, they are still in the early stages of development, and prospective trials are needed to evaluate their use in clinical trials of other immunotherapies in different cancer types and to further investigate the potential association with survival. Recently, the use of the irRC using unidimensional measurements was evaluated (Table 3).77,78 Unidimensional measurements are advantageous as they are simpler and more reproducible, with less chance for misclassification of response. The study indicated that irRC using unidimensional measurements produced a very similar evaluation of response to bidimensional measurements, but with significantly less variability.77
Proven Clinical Rationale for Targeting Immunotherapy in Cancer
Immunotherapy for the treatment of cancer has evolved alongside our improved understanding of the immune system. In particular, an appreciation of the ability of cancer cells to subvert the antitumor immune response has provided a rationale for the development of novel immunotherapies that target immune checkpoints responsible for the regulation of T-cell activity.
Ipilimumab, a mAb targeting CTLA-4, was the first to receive regulatory approval from the FDA. In addition, several agents that target the PD-1 receptor and PD-L1 are being developed, 2 having already received marketing approval (pembrolizumab in the United States and nivolumab in Japan). Development of these agents also provides the opportunity for combination therapy with ipilimumab (and other types of immunotherapy or targeted cancer agents), and reports indicate that this may generate impressive responses in patients with a range of different cancer types.21,23
Figure 3. PD-1 Blockade: Binding to PD-L1 and PD-L281
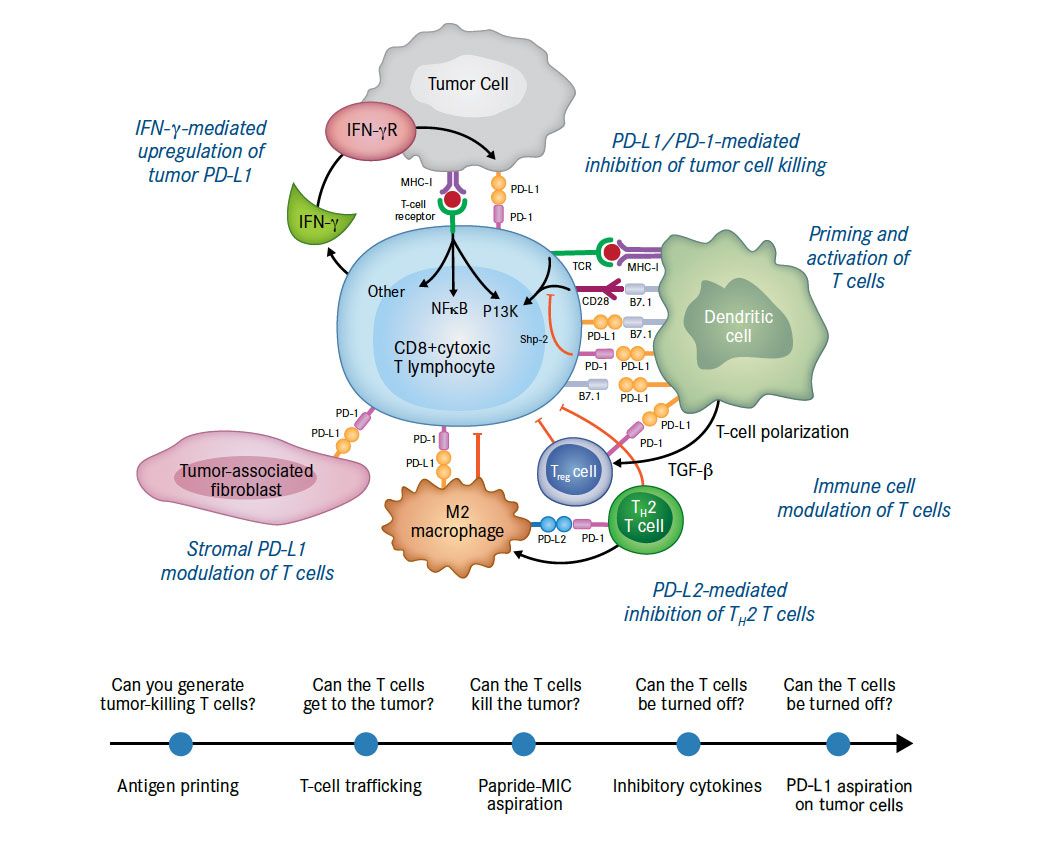
Programmed death-1 (PD-1) is a T-cell molecule that binds to programmed death ligand-1 (PD-L1) or programmed death ligand-2 (PD-L2). PD-L1 is typically expressed on tumor cells and is induced by gamma interferon secreted by activated T cells. The activated T cells that could kill tumors are specifically disabled by those tumors that express PD-L1 and bind to PD-1 to create a phenotype known as T-cell exhaustion.
Adapted from Sznol M, Chen L. Clin Cancer Res. 2013;19(5):1021-1034.
The clinical rationale for targeting the PD-1/PD-L1 pathway is sound. PD-1 is a T-cell molecule that binds to the ligands PD-L1 or PD-L2. PD-L1 is typically expressed on tumor cells and is induced by gamma interferon secreted by activated T cells (Figure 3).81 In brief, the activated T cells that could kill tumors are specifically disabled by those tumors that express PD-L1, which binds to PD-1, and creates a phenotype known as T-cell exhaustion. Clinical data from studies of antibodies directed against PD-1 and PD-L1 have shown encouraging safety profiles and remarkable antitumor activity in subsets of patients with metastatic disease, including malignancies (such as lung cancer) that were previously thought to be unresponsive to immunotherapy.
Continued development of immune-related response criteria that more accurately reflect the unique responses observed with the anti-PD-1/anti-PD-L1 class of drugs will also help to further assist in their clinical evaluation.
References
- American Cancer Society. Cancer facts and figures 2011. American Cancer Society website. http://www.cancer.org/ acs/groups/content/@epidemiologysurveilance/documents/ document/acspc-029771.pdf. Accessed October 30, 2013.
- Coley WB. The treatment of inoperable sarcoma with the mixed toxins of erysipelas and bacillus prodigiosus: immediate and final results in one hundred and forty cases. JAMA. 1898; XXXI(9):456-465.
- Kirkwood JM, Butterfield LH, Tarhini AA, et al. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62(5):309-335.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57-70.
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24): 6580-6587.
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480-489.
- Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757-1766.
- Topalian SL, Drake CG, Pardoll DM, et al. Targeting the PD-1/ B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207-212.
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237-251.
- Helfand C. Top 10 best-selling cancer drugs of 2013. Fierce- Pharma website. http://www.fiercepharma.com/specialreports/ top-10-best-selling-cancer-drugs-2013. Published May 29, 2014. Accessed September 10, 2014.
- Rituxan [package insert]. South San Francisco, CA: Genentech, Inc; 2014.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792.
- Bilusic M, Madan RA. Therapeutic cancer vaccines: the latest advancement in targeted therapy. Am J Ther. 2012;19(6): e172-e181.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
- Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10(5):345-352.
- Ascierto PA, Marincola FM. What have we learned from cancer immunotherapy in the last 3 years? J Transl Med. 2014;12:141.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
- Chen DS, Mellman I. Oncology meets immunology: the cancerimmunity cycle. Immunity. 2013;39(1):1-10.
- Yervoy [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
- FDA approves Keytruda for advanced melanoma: first PD-1 blocking drug to receive agency approval [press release]. US Food and Drug Administration website. http://www.fda .gov/NewsEvents/Newsroom/PressAnnouncements/ ucm412802.htm. Updated September 2014. Accessed September 30, 2014.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-deathreceptor- 1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109-1117.
- Bristol-Myers Squibb and Ono Pharmaceutical Co., Ltd. announce strategic immuno-oncology collaboration in Japan, South Korea and Taiwan [press release]. Bristol-Myers Squibb website. http://news.bms.com/press-release/rd-news/bristol- myers-squibb-and-ono-pharmaceutical-co-ltd-announcestrategic- immuno-o. Bristol-Myers Squibb website. Accessed September 30, 2014.
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336-347.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Brahmer JR. Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol. 2013;31(8): 1021-1028.
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111-122.
- Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517-2519.
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166-182.
- Wang SF, Fouquet S, Chapon M, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS One. 2011;6(3):e17621.
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793-800.
- Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094-5100.
- Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110(1):296-304.
- Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537-1544.
- National Institutes of Health. ClinicalTrials.gov website. https://clinicaltrials.gov.
- Garon EB, Balmanoukian A, Hamid O, et al. Preliminary safety and activity of MK-3475 monotherapy for the treatment of previously treated patients with non-small cell lung cancer (NSCLC). Presented at: 15th World Conference on Lung Cancer; October 27-31, 2013; Sydney, Australia. Abstract MO18.02.
- Ribas A, Robert C, Daud A, et al. Clinical efficacy and safety of lambrolizumab (MK-3475, Anti-PD-1 monoclonal antibody) in patients with advanced melanoma. Presented at: 2013 Annual Meeting of the American Society of Clinical Oncology; May 31-June 4, 2013; Chicago, IL. Abstract 9009.
- Kefford R, Ribas A, Hamid O, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 3005.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134-144.
- Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL). Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract LBA9000.
- Rizvi NA. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2014;32:5s. Abstract 8007.
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of singleagent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167-3175.
- Hamid O, Robert C, Ribas A, et al. Randomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and IPI-naive (IPI-N) melanoma (MEL). Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 3000.
- Topalian SL, Sznol M, Brahmer JR, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with advanced solid tumors: survival and long-term safety in a phase I trial. Presented at: 2013 Annual Meeting of the American Society of Clinical Oncology; May 31-June 4, 2013; Chicago, IL. Abstract 3002.
- Brahmer JR, Horn L, Antonia SJ, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with non-small cell lung cancer (NSCLC): overall survival and long-term safety in a phase I trial. Presented at: 15th World Conference on Lung Cancer; October 27-31, 2013; Sydney, Australia. Abstract MO18.03.
- Hodi FS, Sznol M, Kluger HM, et al. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO- 4538) in a phase I trial. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 9002.
- Brahmer JR, Horn L, Gandhi L, et al. Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced nonsmall- cell lung cancer (NSCLC): survival and clinical activity by subgroup analysis. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 8112.
- Rizvi NA, Antonia SJ, Chow LQM, et al. A phase I study of nivolumab (anti-PD-1; BMS-936558,ONO-4538) plus platinum-based doublet chemotherapy (PT-doublet) in chemotherapy- naive non-small cell lung cancer (NSCLC) patients (pts). Presented at: 2013 Annual Meeting of the American Society of Clinical Oncology; May 31-June 4, 2013; Chicago, IL. Abstract 8072.
- Sznol M, Kluger HM, Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract LBA9003^.
- Hammers HJ, Plimack ER, Infante JR, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 4504.
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): results of a randomized, dose-ranging phase II trial. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 5009.
- Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 5010.
- Rizvi NA, Chow LQM, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 8022.
- Weber JS, Kudchadkar RR, Gibney GT, et al. Updated survival, toxicity, and biomarkers of nivolumab with/without peptide vaccine in patients naive to, or progressed on, ipilimumab (IPI). Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 3009.
- Hamanishi J. Mandai M, Ikeda T, et al. Efficacy and safety of anti-PD-1 antibody (Nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 5511.
- Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase ll trial. Lancet Oncol. 2014;15(1):69-77.
- Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199-4206.
- Herbst RS, Gordon MS, Fine GD, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. Presented at: 2013 Annual Meeting of the American Society of Clinical Oncology; May 31-June 4, 2013; Chicago, IL. Abstract 3000.
- Soria JC, Cruz C, Bahleda R, et al. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1). Presented at: European Cancer Congress; 2013; Amsterdam. Abstract 3408.
- Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol. 2013;31(suppl). Abstract 9010.
- Powles T, Vogelzang NJ, Fine GD, et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC). Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 5011.
- Segal NH. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 3002^.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465.
- Weber JS, Minor DR, D’Angelo SP, et al. A phase 3 randomized, open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus investigator’s choice chemotherapy (ICC) in patients with advanced melanoma after prior anti-CTLA-4 therapy. Presented at: 2014 Annual Meeting of the European Society for Medical Oncology; September 26-30, 2014; Madrid, Spain. Abstract LBA3_PR.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2): 122-133.
- Bristol-Myers Squibb. One- & two-year survival rates of 94% and 88% announced from phase 1b trial of investigational PD-1 checkpoint inhibitor nivolumab and Yervoy® (ipilimumab) in advanced melanoma; ongoing phase 2/3 trials to confirm results. Bristol-Myers Squibb website. http://news .bms.com/press-release/rd-news/one-two-year-survivalrates- 94-and-88-announced-phase-1b-trial-investigational &t=635373988489866086. Accessed September 30, 2014.
- Weber JS, Kudchadkar RR, Gibney GT, et al. Phase I/II trial of PD-1 antibody nivolumab with peptide vaccine in patients naive to or that failed ipilimumab. Presented at: 2013 Annual Meeting of the American Society of Clinical Oncology; May 31-June 4, 2013; Chicago, IL. Abstract 9011.
- Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10): 3044-3051.
- Atkins MB, Kudchadkar RR, Sznol M, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 9001.
- Lutzky J, Antonia SJ, Blake-Haskins A, et al. A phase 1 study of MEDI4736, an anti—PD-L1 antibody, in patients with advanced solid tumors. Presented at: 2014 Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2014; Chicago, IL. Abstract 3001.
- AstraZeneca initiates phase III immunotherapy study for MEDI4736 in patients with lung cancer [press release]. Astra- Zeneca Global website. http://www.astrazeneca.com/Media/ Press-releases/Article/20140508--astrazeneca-initiates-phaseiii- immunotherapy-study-MEDI4736. Accessed October 1, 2014.
- Kohrt H, Kowanetz M, Gettinger S, et al. Intratumoral characteristics of tumor and immune cells at baseline and ontreatment correlated with clinical responses to MPDL3280A, an engineered antibody against PD-L1. J Immunother Cancer. 2013;1(suppl 1):O12.
- WHO handbook for reporting results of cancer treatment. World Heath Organization website. http://apps.who.int/iris/ bitstream/10665/37200/1/WHO_OFFSET_48.pdf?ua=1. Accessed October 1, 2014.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205-216.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
- Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388-1397.
- Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936-3943.
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412-7420.
- Weber J, Thompson JA, Hamid O, et al. A randomized, doubleblind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598.
- O’Day SJ, Ibrahim R, DePril V, et al. Efficacy and safety of ipilimumab induction and maintenance dosing in patients with advanced melanoma who progressed on one or more prior therapies. J Clin Oncol. 2008;26(15S). Abstract 9021.
- Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19(5):1021-1034.




