Previously Treated Myeloma: Making Sense of Plethora of New Options
Multiple myeloma has seen significant treatment advances over the past decade. In 2015 alone, the FDA approved 5 new agents or regimens for previously treated patients.
Rafael Fonseca, MD

Rafael Fonseca, MD
Multiple myeloma has seen significant treatment advances over the past decade. In 2015 alone, the FDA approved 5 new agents or regimens for previously treated patients. Many of these agents were the first of their kind approved in this setting, making for a truly record-breaking year. Approvals included panobinostat (Farydak), the first histone deacetylase inhibitor; daratumumab (Darzalex) and elotuzumab (Empliciti), the first monoclonal antibodies for the tumor type; a new carfilzomib (Kyprolis) combination regimen; and ixazomib (Ninlaro), the first oral proteasome inhibitor.
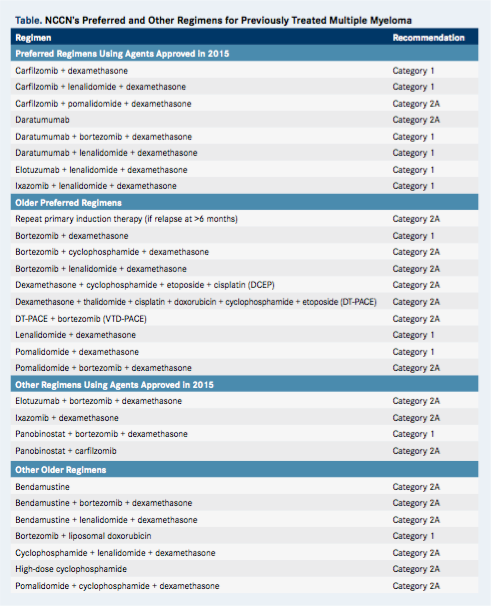
There is no doubt that these approvals are enabling people with multiple myeloma to live significantly longer, but determining how to best use them in clinical practice remains a challenge. The treatment options for previously treated multiple myeloma are abundant; the latest National Comprehensive Cancer Network (NCCN) Guidelines include 18 preferred regimens and 11 other regimens, many of which are category 1(Table).1
During a recent OncLive Peer Exchange panel titled “New Therapeutic Approaches for Multiple Myeloma,” experts in the field sought to make sense of the enhanced treatment terrain.
Deciding on Treatment
“I think we can go through anecdotes, each one of us, why in specific circumstances each of [our available] agents is going to be useful,” said Rafael Fonseca, MD. “All of what we’re discussing are real-world reasons why we may go with one versus another, and I think those real-world reasons are fundamental in how we choose different regimens for our patients.”Although numerous factors can be considered in deciding treatment, including logistical and insurance factors, preferences, comorbidities, and toxicity profiles, the panelists agreed that a major factor in treatment selection for all patients with relapse is the type of relapse.
Elotuzumab and Daratumumab
Patients can have slow, indolent relapses or aggressive relapses. In the setting of aggressive relapse, the consensus was that more powerful therapies need to be used, such as carfilzomib. In contrast, they suggested that indolent relapses enable more flexibility in approach, including watch and wait until symptoms manifest, rather than treating upon observing biochemical progression; initiating more indolent treatments, such as an elotuzumab-based regimen; or proceeding with aggressive treatment, such as a carfilzomib-based therapy.For patients with a relapse that is slow and indolent, “there’s a unique opportunity to incorporate elotuzumab, which targets SLAMF7 expressed on plasma cells as well as NK cells,” said Jatin P. Shah, MD. “One of the exciting things about targeting an immune-stimulating component, the NK cells, is that you potentially can get long-term disease control.”
Elotuzumab is approved in combination with lenalidomide and dexamethasone, an antiangiogenic agent and a corticosteroid, respectively, for patients who previously received 1 to 3 prior medications.2 Approval was based on a trial that showed a progression-free survival (PFS) of 19.4 months for elotuzumab plus lenalidomide and dexamethasone versus 14.9 months for lenalidomide and dexamethasone alone. Additionally, more patients receiving elotuzumab had complete or partial tumor shrinkage (78.5% vs 65.5%).2
However, the trial did not include patients who relapsed after lenalidomide maintenance. “We really don’t know how true lenalidomide-refractory patients would do by adding in elotuzumab,” said William I. Bensinger, MD, who explained he would only consider it for patients who previously responded to lenalidomide.
The other recently approved monoclonal antibody, daratumumab, which targets the CD38 surface protein on myeloma cells, generated considerable excitement among the panelists. “If I’m going to talk about daratumumab, I’m going to say, ‘hang on to your seats,’ because it’s going to revolutionize how we treat myeloma,” said Fonseca.
Unlike elotuzumab, daratumumab can be used alone or in combinations with bortezomib/ dexamethasone or lenalidomide/dexamethasone in patients who have received previous lines of therapy.1 On November 21, the FDA approved both triplet regimens in the relapse setting following at least 1 prior therapy.
“The bottom line is that it works very well... particularly the combinations are quite impressive,” said Fonseca, noting that the depth of response in patients who have not previously responded to any agents sets it apart. He anticipates that daratumumab will move into the frontline setting.
Single-agent daratumumab received accelerated approval based on its response rates in the SIRIUS study.3 Patients who had received a median of 5 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, had an overall response rate (ORR) of 29.9%, with stringent complete response (sCR) in 2.8%, very good partial response (VGPR) in 9.4%, and partial response in 17% of patients. The median duration of response was 7.4 months.3
“The impressive finding is not so much that there was an almost 30% response rate, but that the overall survival (OS) in these heavily pretreated patients was almost 18 months,” said Keith Stewart, MB ChB, explaining that OS in other studies has been closer to 6 months.
The daratumumab combinations received category 1 designations from the NCCN based on the results of several phase III trials.1 When daratumumab was added to bortezomib and dexamethasone, the ORR was 82.9% (vs 63.2% without), the VGPR was 59.2% (vs 29.1% without), and the CR was 19.2% (vs 9.0% without). Similar results were observed when daratumumab was added to lenalidomide and dexamethasone, with an ORR of 92.9% with the triplet versus 76.4% for the doublet. The CR was also significantly improved at 43.1% (vs 19.2% without).1
“My readout is that three-drug combinations for relapse are better than two,” said C. Ola Landgren, MD, PhD. “I think daratumumab in combination with an immunomodulator and dexamethasone definitely could be a rescue for many patients in the future.”
Ixazomib and Carfilzomib
Trials adding ixazomib or carfilzomib to lenalidomide and dexamethasone have also shown benefits. In the TOURMALINE-MM1 trial, which led the FDA to approve ixazomib in combination with lenalidomide and dexamethasone in patients who received at least 1 prior therapy, patients receiving ixazomib had a 35% improvement in PFS (20.6 months vs 14.7 months without).4 Additionally, the ORR was 78.3% and the median duration of response was 20.5 months for the triplet versus 71.5% and 15 months, respectively, for the doublet.4
However, because the trial excluded patients who were on lenalidomide maintenance and progressed, as was also the case with the elotuzumab trial, it is unclear whether this agent can be used in these patients, explained Bensinger, indicating he would consider it for patients who previously responded to lenalidomide.
Unlike the elotuzumab and TOURMALINE-MM1 studies, the ASPIRE trial, which assessed the addition of car lzomib to lenalidomide plus dexamethasone, included patients who were on lenalidomide maintenance and progressed.5 In the study, patients receiving the triplet had a median PFS of 26.3 months and an ORR of 87.1% compared with a PFS of 17.6 months and ORR of 66.7% in those receiving the doublet. Additionally, the carfilzomib group reported superior health-related quality of life.5
“My number one approach has been carfilzomib, lenalidomide, and dexamethasone for first relapse,” said Fonseca, who indicated he would consider the regimen even in those who progressed while on lenalidomide maintenance based on the ASPIRE data.
However, significant benefit has also been observed with adding carfilzomib to pomalidomide (Pomalyst) and dexamethasone, providing another option for patients who are refractory to lenalidomide and bortezomib. A study assessing this triplet reported an ORR of 50%, with 16% achieving VGPR, 34% achieving partial response, 16% achieving minimal response, and 25% having stable disease.6
Panobinostat
“If I have someone who has been on lenalidomide and is failing rapidly and explosively, I would probably go to a triplet of carfilzomib, pomalidomide, and dexamethasone,” said Bensinger.Of the newly approved drugs in 2015, panobinostat was the first, and there was uncertainty among the panelists on when to use it, with some not having much experience with it. It is also the only agent among those approved in 2015 that is not part of an NCCN preferred regimen.
One challenge the panelists noted is that it is an oral drug approved for use with bortezomib, a parenteral drug, which could have led to dosing challenges in clinical trials. The panelists also expressed concern over the toxicity of the panobinostat/bortezomib/dexamethasone triplet regimen.
“There was a fair amount of GI toxicity and I’ve heard of regimens where instead of giving the drug every week, you give it every other week, which seems to be better tolerated,” said Bensinger.
The drug has also been associated with severe and sometimes fatal cardiovascular events. Because of these toxicity concerns, it is approved for use with a risk evaluation and mitigation strategy. Yet despite these challenges, the panelists urged providers not to overlook this drug. “It’s still an active drug, and for an incurable disease, that’s an important option for patients,” said Shah.
Approval of the panobinostat triplet was based on a study that showed a PFS of 10.6 months with the triplet versus 5.8 months with the doublet.7 Patients receiving the triplet also had a 59% response rate compared with a 41% response rate for the doublet.7 In patients previously treated with an immunomodulatory agent and a proteasome inhibitor, the results have been particularly promising.
“The PFS improved from 4 months to 12 months, which was actually a significant improvement in this subset of patients,” said Shah. He noted that other trials are examining panobinostat in combination with other agents, including carfilzomib and lenalidomide, and are showing significantly less toxicity.
Take-Home Message
“I think, globally, we’re now seeing multiple data sets that when you combine panobinostat with carfilzomib or lenalidomide it’s an active combination that’s well tolerated, which is a very different panorama than when you combine it with weekly bortezomib,” he said.“Right now we are at a point where we can deliver deep, quick, and sustained freedom from disease, which is fantastic for patients,” said Landgren. However, because no cure yet exists, the panelists emphasized considering and exhausting all treatment options to optimize outcomes.
When considering treatments, whether for indolent or aggressive disease, they felt numerous non-traditional outcome measures and factors need to be considered, particularly as patients continue to live longer and quality-of-life issues take on increasing importance. “It’s no longer just about median PFS and response rate,” said Shah. “There are a lot more factors that we have to look at—hazard ratio, the shape of the Kaplan-Meier curves, and long-term survival.”
Additionally, with other promising treatments and combinations under development or in clinical trials, the treatment landscape is likely to become even more complex. Subsequently, the panelists felt it was important for community oncologists to turn to multiple myeloma experts for help when needed.
“We realize myeloma is only but a small fraction of your practice and it’s gotten incredibly complex, so we’re happy to partner with you in the care of these patients,” said Fonseca.
References
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Updated October 28, 2016. Accessed November 8, 2016.
- US Food and Drug Administration. FDA approves Empliciti, a new immune-stimulating therapy to treat multiple myeloma [news release]. November 30, 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm474684.htm. Accessed November 8, 2016.
- DARZALEX® (daratumumab) approved by U.S. FDA: first human anti-CD38 monoclonal antibody available for the treatment of multiple myeloma [news release]. Horsham, PA: Janssen Biotech, Inc; November 8, 2016. https://www.darzalex.com/shared/product/darzalex/darzalex-press-release.pdf. Accessed November 8, 2016.
- Takeda presents data from phase 3 TOURMALINE-MM1 study for NINLARO® (ixazomib), first and only once-weekly oral proteasome inhibitor recently approved for multiple myeloma [news release]. Orlando, FL: Takeda Pharmaceutical Company Ltd; December 6, 2015. https://www.takeda.com/news/2015/20151207_7238.html. Accessed November 8, 2016.
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al; ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142-152. doi:10.1056/NEJMoa1411321.
- Combination of carfilzomib, pomalidomide, and dexamethasone well tolerated in patients with relapsed/refractory multiple myeloma. ASH Clinical News. http://ashclinicalnews.org/combination-of-carfilzomib-pomalidomide-and-dexamethasone-well-tolerated-in-patients-with-relapsedrefractory-multiple-myeloma. Updated October 28, 2015. Accessed November 9, 2016.
- Panobinostat. US Food and Drug Administration. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm435339.htm. Updated February 24, 2015. Accessed November 11, 2016.




