Clinicians Turn Their Focus to pMMR Disease Following Immunotherapy’s Entrance Into dMMR Endometrial Cancer
Exploration continues for the use of immunotherapy in patients with mismatch repair–proficient endometrial cancer.
Ritu Salani, MD

With a heavily shifting treatment paradigm as of late, all eyes are on the endometrial cancer landscape, especially concerning the subset of patients with mismatch repair–proficient (pMMR) disease, as several advancements have ushered in immunotherapy for those with recurrent/advanced mismatch repair–deficient (dMMR) or microsatellite instability–high (MSI-H) disease.1
“An interesting area of research is pMMR endometrial cancer—how do we stratify some of these patients [by] looking at subgroup analyses and figuring out the best strategies? We know even with immunotherapy, there still is a high rate of recurrent disease. Looking at new approaches in that setting is what I’m most keen on,” Ritu Salani, MD, the director
of gynecologic oncology and the Gynecologic Oncology Fellowship at UCLA Health in Los Angeles, California, said in an interview with OncologyLive®.
The immunotherapies dostarlimab-gxly (Jemperli) and pembrolizumab (Keytruda) have demonstrated promise in endometrial cancer, with pembrolizumab plus lenvatinib (Lenvima) holding an indication for the treatment of patients with pMMR disease. For those with dMMR disease, a couple of agents are available: Single-agent dostarlimab is approved for adults with recurrent or advanced disease who progressed on or following a platinum-containing regimen, and pembrolizumab is indicated for patients who are not candidates for curative surgery or radiation with MSI-H or dMMR disease that has progressed on prior systemic therapy in any setting.2
Additionally, dostarlimab gained 2 approvals in 2023 for patients with dMMR recurrent or advanced endometrial cancer, first providing an option as monotherapy for adults who are not candidates for curative surgery or radiation and experienced disease progression on or following a prior platinum-containing regimen in any setting.3 The agent was
also granted approval in combination with carboplatin and paclitaxel, followed by single-agent dostarlimab, for primary advanced or recurrent endometrial cancer that is dMMR or MSI-H.4
Pembrolizumab also has the potential to be the first immunotherapy approved in the front-line setting for patients with endometrial cancer regardless of MMR status; a supplemental biologics license application seeking approval of the agent in combination with carboplatin and paclitaxel followed by single-agent pembrolizumab for the treatment of patients with primary advanced or recurrent endometrial carcinoma was accepted by the FDA for priority review. The Prescription Drug User Fee Act date is June 21, 2024.5
Examining the Role of Dostarlimab and Pembrolizumab in pMMR Disease
Homing in on pMMR disease, a press release published in December 2023 revealed that data from part 2 of the phase 3 RUBY trial (NCT03981796) demonstrated that dostarlimab plus chemotherapy followed by dostarlimab plus niraparib (Zejula) significantly improved progression-free survival (PFS) vs chemotherapy alone in patients with pMMR endometrial cancer, meeting the primary end point of the study.6
“I’m excited about understanding some of the nuances of the use of immunotherapy in endometrial cancer,” Salani said. “[For example,] how do we sequence immunotherapy and which patients is it best for up-front vs using it in recurrent lines.”
The FDA approval of pembrolizumab plus lenvatinib for those with pMMR advanced endometrial carcinoma who are not candidates for curative surgery or radiation and have experienced disease progression following prior systemic therapy in any setting was based on data from the phase 3 study KEYNOTE-775 (NCT03517449).7
The final prespecified overall survival (OS) analysis revealed that improved efficacy was seen regarding OS (HR, 0.70; 95% CI, 0.58-0.83) in patients with pMMR advanced, recurrent, or metastatic endometrial cancer treated with the combination of pembrolizumab and lenvatinib (n = 346) vs investigator’s choice of either doxorubicin or paclitaxel (n = 351). The median PFS was 6.7 months (95% CI, 5.6-7.4) vs 3.8 months (95% CI, 3.6-5.0), respectively (HR, 0.60; 95% CI, 0.50- 0.72), and the respective overall response rates (ORRs) were 32.4% vs 15.1%.8 (Table).8,9
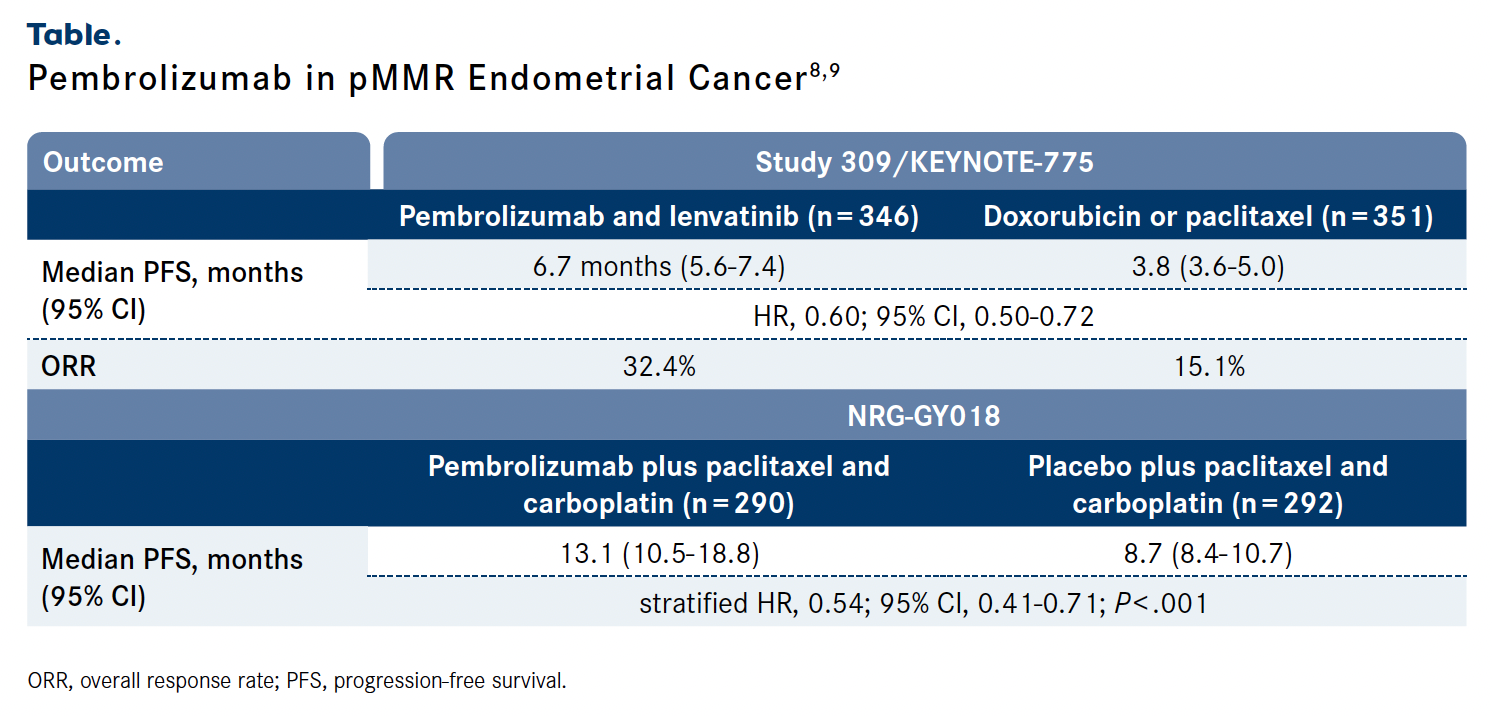
The addition of pembrolizumab to paclitaxel and carboplatin also improved PFS in the phase 3 NRG-GY018 study (NCT03914612) for this patient population. At a median follow-up of 7.9 months, the median PFS was 13.1 months (95% CI, 10.5-18.8) with the combination (n = 290) vs 8.7 months (95% CI, 8.4-10.7) with placebo plus chemotherapy (n = 292; stratified HR, 0.54; 95% CI, 0.41-0.71; P < .001).9
However, Salani noted that although immunotherapy remains a promising area of investigation, recurrent disease remains an area of uncertainty. “If a patient recurs, what do we do next? What’s the next best therapy for these patients? This can be a very challenging disease to treat and finding those best therapies in the second-line and third-line settings continues to be a challenge,” she explained.
dMMR Disease: Questions May Have Been Answered for Now
The United Kingdom has followed suit in advising that patients with dMMR disease receive immunotherapy; a final draft guidance published by the National Institute for Health and Care Excellence recommended the use of dostarlimab in combination with platinum-containing chemotherapy as a treatment for patients with advanced or recurrent endometrial cancer that is MSI-H or dMMR.10
“We changed the standard of care for endometrial cancer,” Salani said, noting that although unanswered questions remain for the pMMR population, the dMMR population appears to be settled regarding optimal treatment approaches. “We had [data from] 4 phase 3 clinical trials that showed the addition of immunotherapy with a checkpoint inhibitor to chemotherapy with maintenance immunotherapy or checkpoint inhibitor use in the dMMR population clearly improved survival outcomes.”
The phase 3 DUO-E trial (NCT04269200) was 1 of the 4 trials whose findings demonstrated a survival advantage for patients treated with immunotherapy followed by maintenance therapy; those with dMMR disease who received durvalumab (Imfinzi) plus olaparib (Lynparza; n = 48) achieved an 18-month PFS rate of 62.7% (95% CI, 46.9%-75.0%), vs 67.9% (95% CI, 51.1%- 80.0%) for those who received durvalumab (n = 46) vs 31.7% (95% CI, 16.7%-47.9%) for the carboplatin/paclitaxel arm (n = 49).
Moreover, patients with pMMR disease achieved 18-month PFS rates of 42.0% (95% CI, 34.1%- 49.6%), 31.3% (95% CI, 24.2%-38.6%), and 20.0% (95% CI, 14.1%-26.7%) in the doublet (n = 191), durvalumab (n = 192), and chemotherapy (n = 192) arms, respectively.11
Findings from part 1 of the RUBY trial, which supported the approval of dostarlimab plus carboplatin and paclitaxel followed by single-agent dostarlimab, showed that treatment with the combination reduced the risk of disease progression or death by 72% vs chemotherapy alone for patients with dMMR disease (HR, 0.28; 95% CI, 0.16-0.50).12 In the phase 1 GARNET trial (NCT02715284), data from which supported the approval of dostarlimab monotherapy in this population, patients with dMMR endometrial cancer who received the agent (n = 141) achieved an ORR of 45.4% (95% CI, 37.0%-54.0%) that comprised a 15.6% complete response rate; the median duration of response was not reached, and 54.7% of patients responded to treatment for at least 24 months.13
Open for Debate: Additional Markers and Subgroups for pMMR
When examining pMMR disease, Salani noted that, “I don’t think we should clump it as just pMMR—it’s a very heterogeneous group. When we look at the pMMR group, the dMMR group may also overlap with this; if a patient has dMMR and then recurs, they would also fall into this bucket where maybe immunotherapy is not the ideal choice if they’ve already been treated with it before.”
She also added that “we have options for p53 wild-type [disease] with selinexor [Xpovio] maintenance. We have a very interesting trial looking at that where the preliminary data have been compelling.”
Regardless of MSI status, patients with TP53 wild-type advanced or recurrent endome- trial cancer experienced improvements in PFS when treated with selinexor maintenance therapy vs placebo in the phase 3 SIENDO/ENGOT-EN5 study (NCT03555422). At a median follow-up of 25.3 months, patients achieved a median PFS of 27.4 months (95% CI, 13.1-not reached) in the selinexor arm (n = 77) vs 5.2 months (95% CI, 2.0-13.1) in the placebo arm (n=36; HR, 0.42; 95% CI, 0.25-0.70; 1-sided nominal P = .0003).14
“Looking at the p53 mutation type and particularly HER2 and looking at the role of trastuzumab [Herceptin]—whether it’s trastuzumab or the antibody-drug conjugate fam-trastuzumab deruxtecan-nxki [Enhertu]— is very interesting,” Salani noted. “There may [also] be other markers such as TROP2 [and] FRα. Understanding when [it’s appropriate] to use these agents, and also the timing of using the agents, [is key]. That is a landscape that’s still to be developed.”
Several trials are underway examining agents in these spaces with investigations focusing on the combination of mirvetuximab soravtan- sine-gynx (Elahere) and pembrolizumab in patients with FRα-positive relapsed serous endometrial cancer. Trastuzumab deruxtecan is also being explored in combination with olaparib in solid tumors.15
Additionally, Salani noted the importance of examining surgical approaches. “We’re understanding that surgeries are a critical part of gynecologic cancer care but it’s not the place where we’re going to make the most progress—that’s with targeted therapies.” She cited research in cervical cancer where advancements have been seen regarding de-escalation of surgical intervention. “[In certain cases], doing a simple hysterectomy or conization may be more appropriate than doing a radical hysterectomy, which has increased surgical morbidity. Offering the best therapy to our patients, not compromising oncologic outcomes but minimizing morbidity, is important,” Salani said. “Studies that are looking at doing less radical surgery can be impactful for patients, and that’s an important change.”
Gynecologic Malignancy Meeting Homes in on Molecularly Targeted Treatments
Noting that pMMR endometrial cancer is an area she’s keen to discuss at the 15th Annual International Symposium on Ovarian Cancer and Other Gynecologic Malignancies™ Meeting, Salani is also excited to discuss cervical cancer at the event, which she is cochairing.16
Sessions including “Navigating Recurrence: Innovative Approaches to Cervical Cancer Management” will do a deep dive into new research in that sector, whereas sessions such as “Medical Crossfire®: Navigating the Management of pMMR in Endometrial Cancer – Debates and Insights” will examine unanswered questions in the field of endometrial cancer. HER2-targeted therapy will also be a focus, and clinicians will have the opportunity to discuss emerging targets following the "Biomarker Testing in Endometrial Cancer: Overview of Current Practices and Future Prospects" presentation.
“I’m looking forward to hearing where the landscape is going for cervical cancer,” Salani said. “We’ve made great strides with the addition of immunotherapy [in gynecologic oncology], but we need to continue to improve the care for patients if they have disease recurrence,” she added.
References
- NCCN. Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1.2024. Accessed March 12, 2024. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Oncology (cancer)/hematologic malignancies approval notifications. FDA. Updated March 7, 2024. Accessed March 12, 2024. bit.ly/4a9JHIt
- FDA grants regular approval to dostarlimab-gxly for dMMR endometrial cancer. FDA. February 9, 2023. Accessed March 12, 2024. bit.ly/3Va1E5r
- FDA approves dostarlimab-gxly with chemotherapy for endometrial cancer. FDA. July 31, 2023. Accessed March 12, 2024. bit.ly/3wYCxs2
- FDA grants priority review to Merck’s application for Keytruda (pembrolizumab) plus chemotherapy as treatment for primary advanced or recurrent endometrial carcinoma. News release. Merck. February 20, 2024. Accessed March 12, 2024. bit.ly/4a9tFhF
- Jemperli (dostarlimab) plus Zejula (niraparib) combination significantly improved progression-free survival in primary advanced or recurrent endometrial cancer in RUBY part 2 phase III trial. News release. GSK. December 18, 2023. Accessed March 12, 2024. bit.ly/3v6ZlFG
- Lenvima. Prescribing information. Eisai Inc; 2023. Accessed March 12, 2024. bit.ly/3PnCMDt
- Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab in previously treated advanced endometrial cancer: updated efficacy and safety from the randomized phase III Study 309/KEYNOTE-775. J Clin Oncol. 2023;41(16):2904-2910. doi:10.1200/JCO.22.02152
- Eskander RN, Sill MW, Beffa L, et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170. doi:10.1056/NEJMoa2302312
- Final draft guidance: dostarlimab with platinum-based chemotherapy for treating advanced or recurrent endometrial cancer with high microsatellite instability or mismatch repair deficiency. National Institute For Health And Care Excellence. February 2024. Accessed March 12, 2024. bit.ly/49Pbeiv
- Westin SN, Moore K, Chon HS, et al; DUO-E Investigators. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: the phase III DUO-E trial. J Clin Oncol. 2024;42(3):283-299. doi:10.1200/JCO.23.02132
- Phase III RUBY trial of Jemperli (dostarlimab) plus chemotherapy meets end point of overall survival in patients with primary advanced or recurrent endometrial cancer. News release. GSK. October 30, 2023. Accessed March 12, 2024. bit.ly/497P9uF
- Jemperli. Prescribing information. GlaxoSmithKline; 2023. Accessed March 12, 2024. bit.ly/4ab1DT6
- Slomovitz B, Pérez Fidalgo A, Hamilton E, et al. Long-term follow up of selinexor maintenance in patients with TP53wt advanced or recurrent endometrial cancer: a prespecified subgroup analysis from the phase 3 ENGOT-EN5/GOG-3055/SIENDO study. J Clin Oncol. 2023;41(suppl 36):427956. doi:10.1200/JCO.2023.41.36_suppl.427956
- Dougherty B. Antibody-drug conjugates — a kind of molecular "smart bomb" — are emerging as an important form of targeted treatment across a variety of cancers. Dana-Farber Cancer Institute. January 11, 2024. Accessed March 12, 2024. bit.ly/4cf1xLM
- 15th Annual International Symposium on Ovarian Cancer and Other Gynecologic Malignancies™ agenda. Physicians’ Education Resource®. Accessed March 12, 2024. bit.ly/3TyMq8Y




