Immune Checkpoint Inhibitors Revolutionize Endometrial Cancer Treatment
The incorporation of immune checkpoint inhibitors has altered the treatment paradigm for endometrial cancer.
Monique A. Spillman, MD, PhD

The International Federation of Gynecology and Obstetrics (FIGO) system characterizes endometrial cancer by grade and stage. Surgery is the main treatment, with chemotherapy or radiation therapy based on stage, patient characteristics, and histology. Modern molecular analysis has further stratified endometrial carcinomas. Loss of mismatch repair (MMR) proteins (MLH1, MSH2, PMS1, or MSH6) results in high microsatellite instability (MSI-H), a hallmark of Lynch syndrome.1
MMR deficient (dMMR) tumors may express PD-L1, which is the cell surface activator of the PD-1 receptor protein. When PD-1 receptors on the surface of T cells bind the PD-L1 ligand on the tumor cell, the tumor cell turns off the killing function of the T cell. However, the killing action of the T-cell–specific response can be reactivated by blocking the PD-L1 protein, or by preventing tumor cell binding at the PD-1 receptor. Immune checkpoint inhibitors (ICI), such as pembrolizumab (Keytruda) and dostarlimab-gxly (Jemperli), include high affinity, high specificity, and humanized monoclonal antibodies to the PD-1 receptor, preventing inactivation of the T cells.
Immunotherapy in Advanced-Stage Endometrial Cancer
According to research results published in several journals, approximately 48% of endometrial carcinomas display PD-L1 positivity, and approximately 25% to 31% are dMMR.2-4 In those tumors, the PD-1 inhibitor pembrolizumab was initially studied in the multicohort phase 1 KEYNOTE-028 trial (NCT02054806).2 This study enrolled 24 patients with advanced-stage, progressive endometrial cancer. PD-L1 expression was determined by immunohistochemistry with 1% positivity in tumor and immune cells denoted as positive. The objective response rate (ORR) by RECIST v1.1 criteria was 13% (95% CI, 2.8%-33.6%) in response-evaluable patients (n = 23). Three patients had partial responses (PR), 3 had stable disease (SD), and 13 experienced progressive disease.2
The phase 2 KEYNOTE-158 study (NCT02628067) investigated pembrolizumab in patients with dMMR or MSI-H advanced solid tumors, including endometrial cancer. Ninety patients with dMMR/MSI-H endometrial cancer were enrolled and received pembrolizumab monotherapy on a 3-week cycle for 35 cycles. Of these patients, 48% had received at least 2 prior lines of therapy, and 68% had received prior radiation. The efficacy analysis was conducted in 79 patients who had received at least 1 dose of pembrolizumab at least 26 weeks prior to data cutoff. An ORR of 48% (95% CI, 37%-60%) was observed, including 11 patients with complete responses, 27 with PRs, and 14 with SD.5 Treatment tolerance was fair, with grade 3 to 4 adverse effects (AEs) reported in 12% of patients in the safety population (n = 90).5
Combining immunotherapy with chemotherapy was the next step. Results of 2 blockbuster trials were published simultaneously in The New England Journal of Medicine in 2023.6,7 In a phase 3 study (NCT03914612), investigators combined pembrolizumab with carboplatin and paclitaxel in all endometrial carcinoma histologies except carcinosarcomas. The randomized, double-blind, placebo-controlled study enrolled 816 patients with stage III to IVB or recurrent endometrial carcinoma. The primary end point was progression-free survival (PFS) and patients were stratified by dMMR or pMMR (MMR proficient) status.6
A significant PFS advantage was observed in the patients with dMMR disease who received pembrolizumab (n = 112) vs placebo (n = 113); the 12-month PFS rates were 74% vs 38%, respectively. The PFS HR was 0.30 (95% CI 0.19-0.48, P < .001) in favor of pembrolizumab in the dMMR cohort. Interestingly, in the pMMR cohort, the median PFS also favored pembrolizumab at 13.1 months (95% CI, 10.5-18.8) vs 8.7 months (95% CI, 8.4-10.7) in the placebo arm (HR 0.54; 95% CI, 0.41- 0.71; P < .001).6
In the phase 3 RUBY trial (NCT03981796), dostarlimab was combined with carboplatin and paclitaxel. The study enrolled patients with recurrent or primary advanced endometrial cancer; carcinosarcomas and other histologies were allowed, and OS was assessed in addition to PFS.7 RUBY was a randomized, placebo-controlled, double-blind study of 494 patients; 23.9% had dMMR/MSI-H disease. In the dMMR/MSI-H cohort, PFS rate at 24 months favored the dostarlimab arm (n = 53) at 61.4% (95% CI, 46.3%-73.4%) vs 15.7% (95% CI, 7.2%-27.0%) in the placebo arm (n = 65; HR, 0.28; 95% CI, 0.16-0.50; P < .001). When patients with pMMR and dMMR disease were both included, the 24-month PFS rate still favored the dostarlimab arm (n = 245) at 36.1% (95% CI, 29.3%-42.9%) vs 18.1% (95% CI, 13.0%-23.9%) in the placebo arm (n = 249; HR, 0.64; 95% CI, 0.51-0.80; P < .001).7 OS rates assessed at 24 months mirrored the PFS rates, favoring dostarlimab at 71.3% (95% CI, 64.5%-77.1%) vs 56.0% (95% CI, 48.9%-62.5%) for placebo (HR, 0.64; 95% CI, 0.46-0.87; P = .0021). Therapy with dostarlimab or placebo was given every 6 weeks for up to 3 years.7
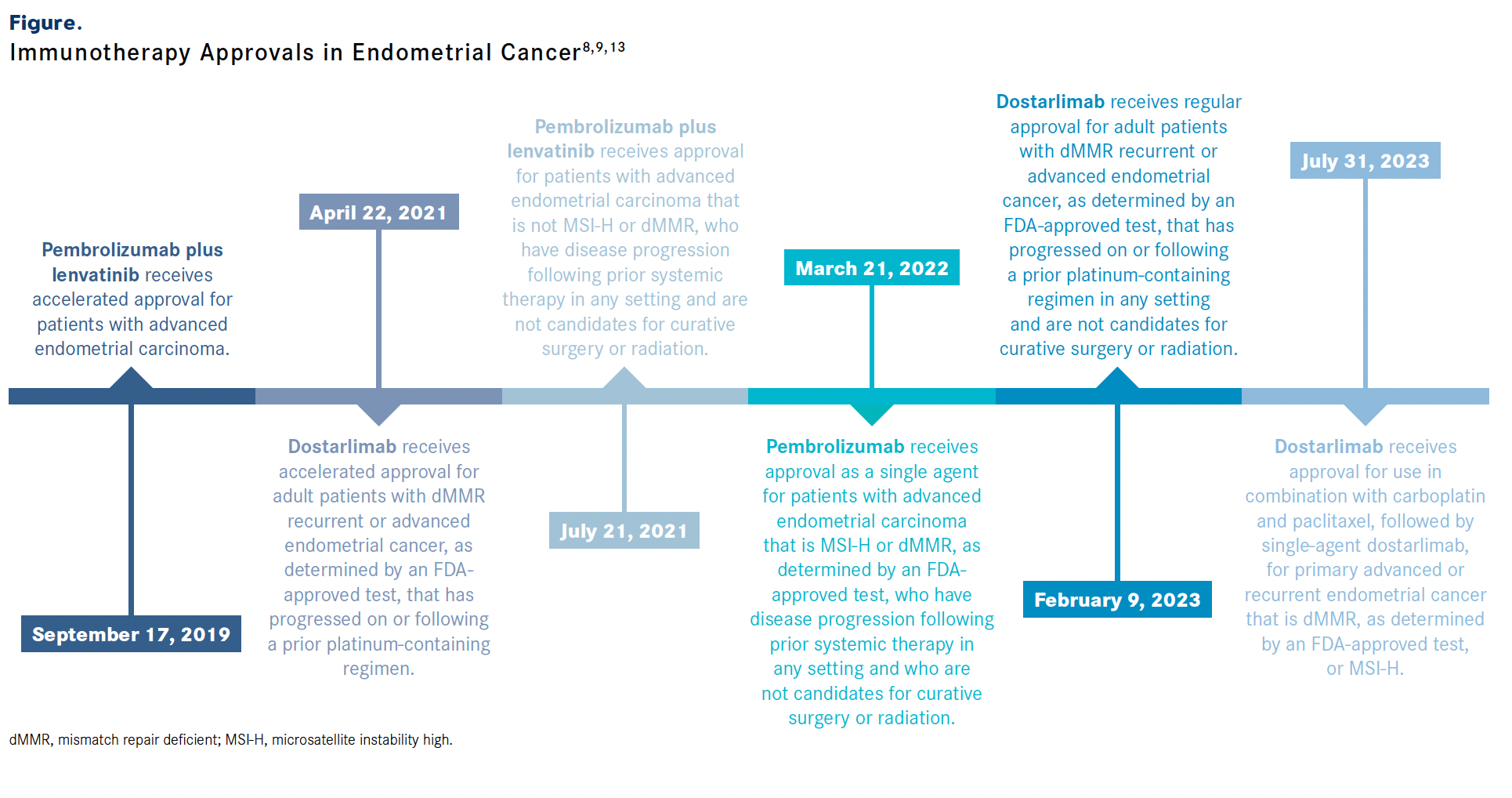
Based on findings from the RUBY trial, the FDA approved dostarlimab in combination with carboplatin and paclitaxel followed by single-agent dostarlimab in advanced or recurrent dMMR or MSI-H endometrial carcinomas.8 Pembrolizumab had previously received FDA approval in March 2022 for use in recurrent endometrial carcinomas that were dMMR/MSI-H.9
Immunotherapy in Recurrent Endometrial Cancer
The use of ICIs in pMMR/microsatellite stable (MSS) endometrial carcinomas has also been investigated in recurrent endometrial cancer. The VEGF angiogenesis pathway is an important target for the modulation of T-cell immune response through tyrosine kinase inhibitors such as lenvatinib (Lenvima). The combination of lenvatinib and pembrolizumab was superior to lenvatinib alone in preclinical models, leading to a phase 1b/2 KEYNOTE-146/Study 111 trial (NCT02501096) in advanced solid tumors.
This trial included 23 patients with endometrial carcinoma, with 48% percent receiving previous systemic therapy in the metastatic setting. Fifty-two percent of the endometrial cancers were PD-L1 positive. Findings from the phase 2 expansion portion demonstrated that the ORR among all patients in the endometrial cancer cohort was 52% (95% CI, 30.6%-73.2%). The median PFS was 9.7 months (95% CI, 4.2-not estimable [NE]). This trial did not specifically test for dMMR or MSI-H status but inferred a potential use in pMMR/MSS tumors from a previous observation that only 25% to 35% of endometrial cancers are dMMR/MSI-H.10
Subsequent findings from the final primary efficacy analysis of the endometrial cancer cohort (n = 108) were stratified by MSI-H/dMMR (n = 11) or MSS/pMMR (n = 94) status. At the median follow-up of 18.7 months (95% CI, 13.1-20.3), the ORR was 63.6% (95% CI, 30.8%-89.1%) in patients with MSI-H tumors and 36.2% (95% CI, 26.5%- 46.7%) in those with MSS tumors. The combined group median duration of response was 21.2 months (95% CI, 7.6-NE), the median PFS was 7.4 months (95% CI, 5.3-8.7), and the median OS was 16.7 months (95% CI, 15-NE). The combination had a 66.9% rate of grade 3 or 4 AEs but displayed a generally manageable toxicity profile.11
In the phase 3 KEYNOTE-775 trial (NCT03517449), patients with platinum-resistant endometrial cancer were randomly assigned to receive pembrolizumab plus lenvatinib or chemotherapy (doxorubicin or weekly paclitaxel). The 827 patients included 697 with pMMR/MSS disease and 130 with dMMR/MSI-H disease, equally divided between 2 arms. Both PFS and OS favored the combination of pembrolizumab and lenvatinib in the overall trial population; the median PFS was 7.2 months (95% CI, 5.7-7.6) vs 3.8 months (95% CI, 3.6-4.2), respectively (HR 0.56; 95% CI, 0.47-0.66; P < .001) and the median OS was 18.3 months (95% CI, 15.2-20.5) vs 11.4 months (95% CI, 10.5-12.9), respectively (HR, 0.62; 95% CI, 0.51-0.75; P < .001).12
In the majority pMMR/MSS group, the results held with both PFS and OS favoring pembrolizumab plus lenvatinib over chemotherapy. pMMR PFS was 6.6 months (95% CI, 5.6-7.4) vs 3.8 months (95% CI, 3.6-5.0), respectively (HR, 0.60; 95% CI, 0.50-0.72; P < .001) and pMMR OS was 17.4 months (95% CI, 14.2-19.9) vs 12.0 months (95% CI, 10.8-13.3), respectively (HR, 0.68; 95% CI, 0.56-0.84; P < .001).12
Based upon results of this trial, the FDA approved the combination of pembrolizumab and lenvatinib in patients with pMMR endometrial cancer with progressive disease who are not candidates for radiation or surgery.13
References
- Berek JS, Matias-Guiu X, Creutzberg C, et al; Endometrial Cancer Staging Subcommittee, FIGO Women’s Cancer Committee. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394. doi:10.1002/ijgo.14923
- Ott PA, Bang Y-J, Berton-Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand-1 positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35(22):2535-2541. doi:10.1200/JCO.2017.72.5952
- Ryan NAJ, Glaire MA, Blake D, Cabrera-Dandy M, Evans DG, Crosbie EJ. The proportion of endometrial cancer associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med. 2019;21(10):2167-2180. doi:10.1038/s41436-019-0536-8
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- O’Malley DM, Bariani GM, Cassier PA, et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol. 2022;40(7):752-761. doi:10.1200/JCO.21.01874
- Eskander RN, Sill MW, Beffa L, et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170. doi:10.1056/NEJMoa2302312
- Mirza MR, Chase DM, Slomovitz BM, et al; RUBY Investigators. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158. doi:10.1056/NEJMoa2216334
- FDA grants regular approval to dostarlimab-gxly for dMMR endometrial cancer. FDA. February 9, 2023. Accessed January 31, 2024. https://bit.ly/3OoswK4
- FDA approves pembrolizumab for advanced endometrial carcinoma 2022. FDA. March 21, 2022. Accessed January 31, 2024. https://bit.ly/3HNhKdo
- Taylor MH, Lee CH, Makker V, et al. Phase Ib/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol. 2020;38(11):1154-1163. doi:10.1200/JCO.19.01598
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38(26):2981-2992. doi:10.1200/JCO.19.02627
- Makker V, Colombo N, Casado Herráez A, et al; Study 309–KEYNOTE-775 Investigators. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437-448. doi:10.1056/NEJMoa2108330
- FDA grants regular approval to pembrolizumab and lenvatinib for advanced endometrial carcinoma. FDA. Updated February 1, 2022. Accessed January 31, 2024. https://bit.ly/49t2Ncm




