Publication
Article
Combination Regimens Stir Excitement in Advanced RCC
Author(s):
Expert oncologists who treat patients with advanced renal cell carcinoma discuss updates from clinical trials presented during the 2023 ESMO Congress.
Benjamin L. Maughan, MD, PharmD

Renal cell carcinoma (RCC) accounted for approximately 90% of the estimated 431,288 new cases of kidney cancer that were diagnosed globally in 2020.1 Although multiple treatment options exist for patients with advanced disease, there remains an unmet need for additional regimens that offer greater efficacy and provide an option for patients in hard-to-treat subgroups, such as those with metastases or poor International Metastatic RCC Database Consortium (IMDC) risk status.
During a recent OncLive Peer Exchange, expert oncologists who treat patients with advanced RCC discussed updates from clinical trials presented during the 2023 European Society for Medical Oncology (ESMO) Congress that further defined the treatment paradigm in advanced RCC and gave a peek into the potential future of the disease management.
Frontline Management of Advanced RCC
Several regimens are available to clinicians to choose from for the frontline treatment of patients with advanced RCC and thus also have multiple factors to consider when selecting the most appropriate regimen. “IMDC [risk score] continues with every study to demonstrate its powerful prognostic capacity,” Benjamin L. Maughan, MD, PharmD, said. “[However], there are some limitations you need to consider. Sites of metastasis are particularly important. Does the patient have an [inferior vena cava] thrombus? That’s not part of the IMDC risk factors and those patients have a particularly poor prognosis, so it’s important to consider the IMDC but, as a clinician in the room with the patient, [to also] consider these other factors.”
Clear Cell RCC Therapeutic Choices Take Steps Forward
During the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, results from the final prespecified overall survival (OS) analysis of the phase 3 CLEAR trial (NCT02811861) were presented. CLEAR enrolled treatment-naive patients with advanced RCC with a clear cell component and randomly assigned them in a 1:1 manner to be treated with either the multireceptor tyrosine kinase inhibitor (TKI) lenvatinib (Lenvima) plus the PD-1 inhibitor pembrolizumab (Keytruda; n = 355) or the TKI sunitinib (Sutent; n = 357).2
At a median follow-up of 49.8 months (range, 41.4-53.1), patients in the combination arm experienced a median OS of 53.7 months (95% CI, 48.7-not estimable [NE]) compared with 54.3 months (95% CI, 40.9-NE) at a median follow-up of 49.4 months (95% CI, 41.6- 52.8) in the sunitinib arm (HR, 0.79; 95% CI, 0.63-0.99; P = .0424). However, the 24- (80.4% vs 69.6%, respectively), 36- (66.4% vs 60.2%), and 48-month (55.9% vs 52.5%) OS rates all favored the combination arm, although the benefit was driven more by the intermediate- and poor-risk (HR, 0.74; 95% CI, 0.57-0.96) subgroup than the favorable-risk (HR, 0.94; 95% CI, 0.58- 1.52) subgroup.2
Additionally, lenvatinib plus pembrolizumab provided a significant benefit in terms of progression-free survival (PFS) vs sunitinib at 23.9 months (95% CI, 20.8-27.7) vs 9.2 months (95% CI, 6.0-11.0), respectively (HR, 0.47; 95% CI, 0.38-0.57; P < .0001), and overall response rate (ORR) at 71.3% (95% CI, 66.6%-76.0%) vs 36.7% (95% CI, 31.7%-41.7%), respectively (relative risk, 1.94; 95% CI, 1.67-2.26; P < .0001).2
“It’s remarkable sitting here today,” Matthew T. Campbell, MD, MS, said. “I started my clinic in 2015 and none of the regimens that we’re discussing were available. To have a regimen that has a median PFS of close to 2 years is quite remarkable. What we got to see over time is additional follow-up, which helps us with OS. The OS [HR] has increased with time and is now close to 0.8. There are a lot of challenges with using OS [to draw conclusions] on these trials where you have multiple later-line options, especially when we’re doing global studies where these options are very different between countries. One of the concerns that may be raised is when you start with such a potent regimen, your later-line options are not going to be as active.”
Safety results from CLEAR showed that any-grade treatment-related adverse effects (TRAEs) occurred in 96.9% and 92.1% of patients in the combination and monotherapy arms, respectively. Grade 3 or higher TRAEs were reported in both arms (74.1% vs 60.3%, respectively), as were deaths (1.1% vs 0.3%). The most common any-grade TRAEs in the investigational arm were diarrhea (56.0%), hypertension (54.3%), hypothyroidism (44.9%), and decreased appetite (35.5%).2
More recently, during the 2023 ESMO Congress, investigators presented findings from a post hoc analysis of CLEAR. The exploratory analysis evaluated the efficacy of lenvatinib plus pembrolizumab vs sunitinib based on patient baseline metastatic characteristics at the final prespecified OS analysis. Patients were stratified based on metastatic site (lung vs lymph node vs bone vs liver vs brain), number of metastatic sites (1 vs ≥ 2), and baseline sums of diameters of target lesions (≥ 60 mm vs < 60 mm).3
At the July 31, 2022, data cutoff, lenvatinib plus pembrolizumab provided a benefit over sunitinib in terms of ORR in every subgroup that was examined. The greatest benefit with the combination was observed in patients with baseline sums of diameters of target legions of at least 60 mm (odds ratio [OR], 10.50; 95% CI, 6.08-18.13), baseline lung metastasis (OR, 5.19; 95% CI, 3.48-7.72), and at least 2 metastatic sites or organs (OR, 5.06; 95% CI, 3.40-7.53).3
Similarly, the combination of lenvatinib plus pembrolizumab provided significant benefit in terms of PFS in every subgroup that was examined. The most pronounced benefit was observed in patients with baseline brain metastasis (HR, 0.32; 95% CI, 0.07-1.59), baseline sums of diameters of target lesions of at least 60 mm (HR, 0.39; 95% CI, 0.29-0.53), and at least 2 metastatic sites/organs (HR, 0.40; 95% CI, 0.31- 0.50; Table 1).3
Table 1. PFS by Subgroups in Post Hoc Analysis of Phase 3 CLEAR Trial3

Updated findings from the 5-year analysis of the phase 3 KEYNOTE-426 trial (NCT02853331), which compared the TKI combination of axitinib (Inlyta) plus pembrolizumab with sunitinib in patients with treatment-naive locally advanced or metastatic clear cell RCC, were also presented during the 2023 ASCO Annual Meeting. At a median follow-up of 67.2 months (range, 60.0-75.0), results from KEYNOTE-426 demonstrated that the median PFS was 15.7 months (95% CI, 13.6-20.2) in the combination arm (n = 432) compared with 11.1 months (95% CI, 8.9-12.5) in the sunitinib arm (n = 429; HR, 0.69; 95% CI, 0.59-0.81). The ORR was 60.6% with an 11.6% complete response (CR) rate compared with an ORR of 39.6% with a 4.0% CR rate, respectively.4
Additionally, the median OS was 47.2 months (95% CI, 43.6-54.8) vs 40.8 months (95% CI, 34.3- 47.5) in the combination arm vs the control arm, respectively (HR, 0.84; 95% CI, 0.71-0.99). When stratified by IMDC risk score, patients with intermediate- and poor-risk disease (HR, 0.76; 95% CI, 0.62-0.93) appeared to derive more benefit from treatment with the combination vs sunitinib than those with favorable risk (HR, 1.10; 95% CI, 0.79-1.54).4
Another phase 3 study, CheckMate 9ER (NCT03141177), is testing a different checkpoint inhibitor combination in clear cell RCC, combining the PD-1 inhibitor nivolumab (Opdivo) with the multireceptor TKI cabozantinib (Cabometyx). The study enrolled patients with advanced clear cell RCC and randomly assigned them in a 1:1 manner to receive either the combination (n = 323) or sunitinib (n = 328).5
Updated findings from the 3-year follow-up of CheckMate 9ER, which were presented during the 2023 ASCO Genitourinary Cancers Symposium, showed that at a median follow-up of 44.0 months and a minimum follow-up of 36.5 months, the median OS was 49.5 months vs 35.5 months in the combination and control arms, respectively (HR, 0.70; 95% CI 0.56- 0.87; P = .0014). Additionally, the median PFS was 16.6 months vs 8.4 months, respectively (HR, 0.59; 95% CI, 0.49-0.71; P < .0001).5
Notably, the combination arm was favored over the control arm in terms of response rate, median PFS, and median OS in nearly every IMDC risk score subgroup that was examined. The most pronounced PFS benefit with nivolumab plus cabozantinib vs sunitinib was observed in patients with IMDC poor-risk disease (HR, 0.37; 95% CI, 0.24-0.57), as was the most significant OS benefit (HR, 0.46; 95% CI, 0.30-0.72; Table 2).5
Table 2. Efficacy Outcomes by IMDC Risk Group in Phase 3 CheckMate 9ER Trial5
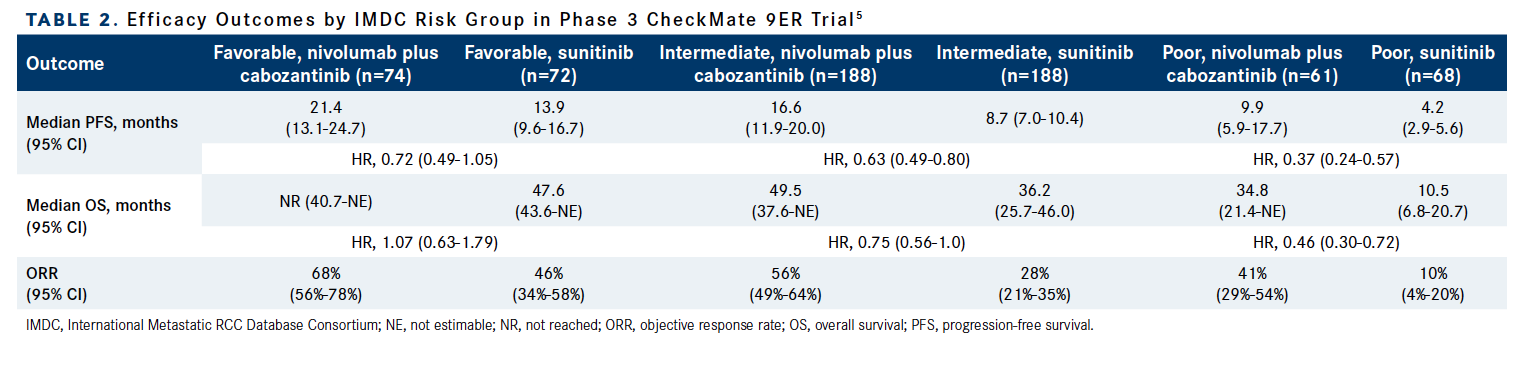
“The combination of cabozantinib [plus] nivolumab is one of the most common combinations [in clinical practice]. I’ve been using it since it was approved a few years ago,” Neeraj Agarwal, MD, said. “The OS continues to be quite strongly maintained with the combination compared with sunitinib. It’s so gratifying to see that median OS of our patients consistently exceeding 4 years. There was a very strong improvement in PFS, which continues to be maintained after almost 4 years of follow-up, with a 50% reduction in risk of progression [or death]. I continue to be very impressed by [these] data.”
KEYNOTE-B61 Aims to Add Option in Non–Clear Cell RCC
Beyond clear cell RCC, the combination of lenvatinib plus pembrolizumab has also shown antitumor activity among patients with treatment- naive advanced non–clear cell RCC. To date, the phase 2 KEYNOTE-B61 trial (NCT04704219) is the largest prospective trial evaluating an immune checkpoint inhibitor combination in non–clear cell RCC.6
“Non–clear cell RCC is a heterogeneous bag of different diseases, with papillary RCC being the most common, but we also have translocation RCC, unclassified RCC, chromophobe RCC, and others,” Laurence Albiges, MD, PhD, said. “The challenge is: How shall we treat them? These tumors don’t have the same biology. However, until a few years ago, we were treating them the same way. We have data supporting the use of VEGF TKIs. [However], with single-agent TKIs, the response rate is somewhere between 5% [and] 20%, and the [median] PFS is quite short—approximately 6 months.”
At the November 7, 2022, data cutoff, findings from KEYNOTE-B61, which were presented during the 2023 ASCO Annual Meeting and subsequently published in Lancet Oncology, revealed that efficacy-evaluable patients who received lenvatinib plus pembrolizumab (n = 158) achieved an ORR of 49% (95% CI, 41%-57%) with a 6% CR rate. The median PFS was 17.9 months (95% CI, 14.0-not reached [NR]) and the median OS was NR (95% CI, NR-NR). The 1-year PFS and OS rates were 63% (95% CI, 54%-70%) and 82% (95% CI, 75%-88%), respectively.6
Notably, the combination displayed antitumor activity regardless of RCC histology. Patients with papillary (n = 93), chromophobe (n = 29), unclassified (n = 21), translocation (n = 6), and other (n = 9) histologies experienced ORRs of 54% (95% CI, 43%-64%), 28% (95% CI, 13%-47%), 52% (95% CI, 30%-74%), 67% (95% CI, 22%-96%), and 56% (95% CI, 21%-86%), respectively.6
In terms of safety, most patients experienced an any-grade AE (99.4%), 65.2% had an AE of grade 3 to 5 severity, and no patients died due to a TRAE. Treatment dose reductions (34.2%), interruptions (72.2%), and discontinuations (15.8%) due to AEs were all reported. The most common any-grade TRAEs consisted of hypertension (57.0%), diarrhea (43.7%), and hypothyroidism (36.7%).6
Investigators concluded that the findings presented at the 2023 ASCO Annual Meeting supported the use of pembrolizumab plus lenvatinib as a frontline treatment option for patients with non– clear cell RCC, irrespective of histology. The safety profile of the combination was manageable and consistent with what has been observed in previous studies of pembrolizumab plus lenvatinib, they added. KEYNOTE-B61 is still active but is not recruiting new patients.6
“The key finding is that the response rate is reaching 50% in this challenging patient population, except for the chromophobe [RCC population], which was a bit lower in the range of 30%,” Albiges said. “The PFS is indeed prolonged with the combination regimen. [Median OS] was NR at the time of the data cutoff, but it’s likely to be above what we’ve had with other combinations in other trials in the past because, at the 1-year mark, 80% of patients were still alive. We are changing the game with combination therapy. Now, in my clinic, a patient [who] is not enrolled in a trial [and] has non–clear cell histology would be considered for combination therapy.”
Future of Advanced RCC
After reviewing multiple case studies pertaining to the management of patients with advanced RCC, the panelists shifted focus to the future and opened their conversation by discussing emerging regimens under investigation in the space.
One regimen that is showing promise in underserved patients with IMDC intermediate- or poor-risk advanced RCC is the triplet combination comprising cabozantinib, nivolumab, and ipilimumab (Yervoy), which is being compared with placebo plus nivolumab and ipilimumab, in patients with clear cell disease in the phase 3 COSMIC-313 trial (NCT03937219). Data from the study, which were published in the New England Journal of Medicine, revealed that patients in the triplet arm (n = 276) achieved a median PFS of NR (95% CI, NR, 14.0-NE) vs 11.3 months (95% CI, 7.7-18.2) among patients in the placebo arm (n = 274). The 12-month PFS rates were 57% (95% CI, 50%-63%) compared with 49% (95% CI, 42%-55%), respectively (HR, 0.73; 95% CI, 0.57-0.94; P = .01).7
Regarding safety, 100% of patients in both the investigational (n = 426) and control arms (n = 424) experienced an any-grade AE. The most common any-grade AEs in the experimental arm included diarrhea (50%), increased alanine aminotransferase levels (49%), and increased aspartate aminotransferase levels (46%). Grade 3 or 4 AEs in the triplet arm occurred at a rate of 79% and included increased alanine aminotransferase levels (27%), increased aspartate aminotransferase levels (20%), and hypertension (10%).7
“Although COSMIC-313 was positive for the primary end point, the toxicity is just a little too much,” Alan Tan, MD, said. “There were a lot of grade 3/4 toxicities, which probably are limiting, and not a lot of patients got the full 4 doses of ipilimumab. So right now, the recommendation is that triplet therapy is not ready for prime time yet.”
During the 2023 ESMO Congress, investigators presented updated findings from multiple clinical trials evaluating doublet regimens and monotherapy strategies in the frontline setting of advanced RCC. The panelists paid special attention to findings from the phase 3 LITESPARK-005 (NCT04195750) and RENOTORCH (NCT04394975) trials.
LITESPARK-005 enrolled patients with advanced clear cell RCC who had received previous treatment with 1 to 3 systemic regimens, including a PD-1/L1 inhibitor and a VEGFdirected TKI, and randomly assigned them 1:1 to receive either belzutifan (Welireg; n = 374) or everolimus (Afinitor; n = 372). At a median follow-up of 25.7 months (range, 16.8-39.1), the median PFS per RECIST v1.1 criteria in both arms was 5.6 months (HR, 0.74; 95% CI, 0.63-0.88); however, the 12- (33.7% vs 17.6%, respectively) and 18-month PFS rates (22.5% vs 9.0%) both favored belzutifan. Notably, the ORRs were 22.7% (95% CI, 18.6%-27.3%) vs 3.5% (95% CI, 1.9%-5.9%), respectively (P < .00001).8
In RENOTORCH, investigators compared the safety and efficacy of toripalimab- tpzi (Loqtorzi) plus axitinib with that of sunitinib for the frontline treatment of patients with advanced RCC. At a median follow-up of 14.6 months, the median PFS in the combination arm (n = 210) was 18.0 months (95% CI, 15.0-NE) vs 9.8 months (95% CI, 8.3-13.8) in the sunitinib arm (n = 211; HR, 0.65; 95% CI, 0.49-0.86; P = .0028). The ORR was 56.7% with a 4.8% CR rate, vs 30.8% with a 3.8% CR rate, respectively.9
“I am incredibly excited about how far we’ve come in terms of treatment, and I am excited that we have a lot of potential targets for cellular therapy that are under exploration,” Campbell said in conclusion.
References
- Bukavina L, Bensalah K, Bray F, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82(5):529-542. doi:10.1016/j.eururo.2022.08.019
- Motzer RJ, Porta C, Eto M, et al. Final prespecified overall survival (OS) analysis of CLEAR: 4-year follow-up of lenvatinib plus pembrolizumab (L+P) vs sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2023;41(suppl 16):4502. doi:10.1200/JCO.2023.41.16_suppl.4502
- Gruenwald V, McKay RR, Buchler T, et al. Tumor response by baseline metastases in patients (pts) with renal cell carcinoma (RCC) treated with lenvatinib (L) plus pembrolizumab (P) vs sunitinib (S): post hoc analysis of the CLEAR trial. Ann Oncol. 2023;34(suppl 2):S1024-S1025. doi:10.1016/j.annonc.2023.09.1133
- Rini BI, Pilmack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma: 5-year analysis of KEYNOTE-426. J Clin Oncol. 2023;41(suppl 17):LBA4501. doi:10.1200/JCO.2023.41.17_suppl. LBA4501
- Burotto M, Powles T, Escudier B, et al. Nivolumab plus cabozantinib vs sunitinib for first-line treatment of advanced renal cell carcinoma (aRCC): 3-year follow-up from the phase 3 CheckMate 9ER trial. J Clin Oncol. 2023;41(suppl 6):603. doi:10.1200/ JCO.2023.41.6_suppl.603
- Albiges L, Gurney H, Atduev V, et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2023;24(8):881-891. doi:10.1016/S1470-2045(23)00276-0
- Choueiri TK, Powles T, Albiges L, et al. Cabozantinib plus nivolumab and ipilimumab in renal-cell carcinoma. N Engl J Med. 2023;388(19):1767-1778. doi:10.1056/NEJMoa2212851
- Albiges L, Rini BI, Peltola K, et al. Belzutifan versus everolimus in participants (pts) with previously treated advanced clear cell renal cell carcinoma (ccRCC): randomized open-label phase III LITESPARK-005 study. Ann Oncol. 2023;34(suppl 2):S1329-S1330. doi:10.1016/j.annonc.2023.10.090
- Sheng X, Ye M, Zou Q, et al. RENOTORCH: toripalimab combined with axitinib versus sunitinib in first-line treatment of advanced renal-cell carcinoma (RCC) - a randomized, open-label, phase III study. Ann Oncol. 2023;34(suppl 2):S1011-S1012. doi:10.1016/j. annonc.2023.09.1112









