Publication
Article
Comprehensive Treatment Advances Care for Patients With Early-Stage Breast Cancer
Author(s):
Advances in the diagnosis and treatment of early-stage breast cancer highlight the need for clinicians to adapt to paradigm shifts that will have notable effects on patient care.
Gwendolyn M. Bryant-Smith, MD

Paradigm shifts in breast cancer treatment necessitate a team approach. Comprehensive treatment provided by a specialized group of experts has become the standard of care in breast cancer. A one-size-fits-all treatment approach no longer works for these patients—it takes a multidisciplinary team and a personalized patient approach to achieve the best outcomes. Advances in the diagnosis and treatment of early-stage breast cancer (ESBC) highlight the need for clinicians to adapt to paradigm shifts that will have notable effects on patient care.
Breast cancers are typically defined by stage and biological behavior. These descriptors aid the multidisciplinary breast team in making care decisions. The primary objective of breast cancer treatment is early detection, which allows for removal of the cancer without metastasis or recurrence.
With advancements and improved knowledge of the biological behavior of breast cancer, the American Joint Committee on Cancer Staging Manual, 8th edition, incorporated additional biological prognostic factors that facilitate predictions of disease severity. These factors include anatomic, clinical, and pathologic biomarkers.1 Size, spread, grading, biomarkers (estrogen receptor, progesterone receptor, HER2), Ki-67, and recurrence score are also evaluated. Understanding stage and biological behavior allows for a better prediction of outcomes and decisions related to treatment.
Stage 0 disease includes ductal carcinoma in situ (DCIS). In situ early cancers are contained within the basement membrane of the duct or lobule. Stage I and IIA define early invasive breast cancers that have penetrated or grown through the basement membrane with low or intermediate grade proliferation rates without spread to regional nodes.
ESBC is a cancer that has not grown outside of the breast and has a low-grade biological profile of stage 0 to IIA. Numerous advances in diagnostic, surgical, and oncologic management of ESBCs have been made. Data from phase 1 prospective basic, translational, and clinical trials to single institutional studies have been published validating impact and advances. Consensus statements and clinical practice guidelines have enhanced the care of patients providing guidelines for prevention through survivorship.2
Screening and diagnostic detection have advanced through the incorporation of digital breast tomosynthesis (DBT), breast ultrasound, and breast MRI. Protein-targeted therapies, including biological response modifiers and hormone-targeted endocrine therapy, continue to be major components of treatment. As the treatment paradigm of breast cancer continues to shift, less aggressive techniques (de-escalation) with targeted therapies may prove comparable to conventional surgery, chemotherapy, and radiation.
Christopher Jean-Louis, DO, MPH

Advances in Screening and Diagnosis: Breast Imaging
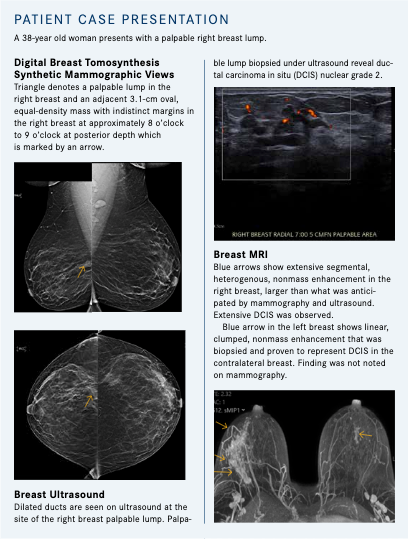
DBT, also known as a 3-dimensional mammogram, is increasingly becoming the first-line standard in mammography breast cancer screening. DBT was approved by the FDA in 2011. Studies consistently have shown that using DBT leads to increased invasive breast cancer detection and decreased call backs and additional work-ups for noncancer. Even with these improvements in mammography, breast density continues to be a limit-ing factor in breast cancer detection, necessitating the need for other supplemental screening tools such as screening breast ultrasound and screening breast MRI.
Breast ultrasound is both a diagnostic and a screening tool. In the diagnostic setting, it is used to further evaluate masses, asymmetries, and architectural distortion noted on mammography. It is also used as a first-line diagnostic tool in female patients younger than 30 years who present with a palpable lump. Ultrasound is helpful in distinguishing between a benign cyst and a suspicious mass.
Notably, breast ultrasound has become very useful in the screening setting in low- and middle-resource countries.3,4 Breast ultrasound is also important as a complementary screening tool to mammography in patients with an elevated lifetime risk of breast cancer who are unable to undergo breast MRI. Additionally, it can be a supplement screening tool for patients with intermediate-risk disease who have dense breast tissue.
Breast ultrasound as a complement to mammography in these patients finds approximately 2 to 4 more cancers per 1000 patients compared with screening mammography alone.5 Digital breast mammography without tomosynthesis typically finds 4.4 to 4.6 cancers per 1000 patients; DBT finds 5.4 cancers per 1000 patients.6,7 Adding breast ultrasound screening to mammography screening has been shown to find 2 to 4 more primarily node-negative invasive breast cancers per 1000 patients.6,7 However, this increase in cancer detection with screening ultra-sound is a trade-off to finding and possibly performing a biopsy for benign disease.
Breast MRI is a complementary imaging study to mammography and is used in both the diagnostic and screening settings for breast cancer detection. MRI not only evaluates the morphology of masses but also the physiology of masses, nonmass enhancement, and foci through gadolinium contrast enhancement.
As a diagnostic tool, it is used to evaluate the extent of disease in patients with newly diagnosed breast cancers and patients’ response to neoadjuvant chemotherapy. In the screening setting, it evaluates high risk patients with a greater than 20% lifetime risk of breast cancer by a mathematical risk assessment model, patients with genetic mutations that predispose them to breast cancer, and patients who have undergone chest irradiation between the ages of 10 to 30 years. Screening breast MRI detects an additional 14 cancers per 1000 patients screened, which is in addition to those noted on mammography.
Treatment Considerations
The 2 mainstay surgical options for the treatment of ESBC are lumpectomy and mastectomy with or without axillary evaluation. One major advance is the utilization of oncoplastic surgical techniques, which have contributed to improved surgical outcomes. Several additional advanced techniques have been implemented for the correction of associated breast defects and reshaping the natural breast contour. These techniques include lumpectomy with tissue rearrangement and therapeutic breast reduction for symmetry.
The round block/Benelli technique for skin reduction provides an approach for cancers located in distant quadrants of the breast. The hidden scar approach is a technique that allows the surgical site to be hidden and yields a superior cosmetic outcome with either lumpectomy or mastectomy.8 In patients receiving mastectomies, the angel wing technique for lateral adiposity allows for flat mastectomies with removal of redundant medial and lateral adipose tissue.9
Some patients with ESBC and a favorable biology may be candidates for deescalation. Over the past 10 years, there has been a major paradigm shift in the management of the axilla in ESBCs, including axillary lymph nodal dissection (ALND) and the elimination of nodal evaluation in patients receiving lumpectomy with a diagnosis of DCIS (stage 0).
In early-stage invasive breast cancer, the ACOSOG Z0011 trial (NCT00003855) demonstrated that omitting ALND with 1 to 2 positive sentinel lymph nodes in patients receiving breast conservation lumpectomy and radiation was not inferior to those receiving ALND. There was no difference in overall survival in these 2 groups.10 The Cancer and Leukemia Group B 9343 stated that patients with a low risk for breast cancer recurrence who were 70 years or older could forgo radiation.11 Genomic test-ing helps predict risk of recurrence and has led to individualized cancer treatments through an understanding of the biologic behavior of breast cancer and its relationship to specific genes.12 Therefore, this reduces overtreatment or undertreatment across the platform of ESBC management.
Ronda S. Henry-Tillman, MD

Survivorship
It is not surprising that mortality rates have steadily fallen in concert with advances in the breast cancer care platform. Currently, more than 90% of patients with ESBC have a projected survival of greater than 5 years.13 However, numerous considerations must be taken into account when assessing this population. These patients remain at risk indefinitely for local recurrence, systemic recurrence, and contralateral new primary breast cancers. In addition, breast cancer survivors also need multidisciplinary care to manage complications or secondary effects from their previous cancer treatments.13,14
Optimizing care for this patient population is critical to the overall health care landscape and has led to greater focus on breast cancer survivorship care. Numerous studies have evaluated the benefit of early detection by circulation tumor biomarkers or advanced imaging on recurrence and survival outcomes. However, there is still no solid correlation. Current data and consensus recommendations from the American Society of Clinical Oncology and the American College of Physicians state that a performance of yearly screening mammography is the only tool supported by evidence from randomized clinical trials to affect mortality and survivorship.15 Other import-ant items such as bone health, fertility/infertility, psychological health, and adherence to screening require a multispecialty team approach and follow-up. Therefore, in our survivorship program, we recommend yearly mammography for patients who have undergone breast conservation and additional tests or imaging based on patients’ symptoms. We must embrace a multidisciplinary team approach and continue to develop personalized care plans for each of our patients with breast cancer.
References
- Amin MB, Edge SB, Greene F, et al, eds. AJCC Cancer Staging Manual, 8th Edition. American Joint Committee on Cancer; 2018.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583-589. doi:10.1016/S1470-2045(13)70134-7
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast can-cer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499-2507. doi:10.1001/jama.2014.6095
- Henry-Tillman R, Kabongo M, Laryea J, et al. The ability to look: management of breast disease in the Democratic Republic of the Congo using smart ultrasound technology. J Am Coll Surg. 2021;232(4):636-640. doi:10.1016/j.jamcoll-surg.2020.12.008
- Rafferty EA, Park JM, Philpotts LE, et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology.2013;266 (1):104-113. doi:10.1148/radiol.12120674
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005; 23(33):8469-8476. doi:10.1200/jco.2004.00.4960
- Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244(2):381-388.doi:10.1148/radiol.2442060461
- Macmillan RD, McCulley SJ. Oncoplastic breast surgery: what, when and for whom?. Curr Breast Cancer Rep. 2016;8:112-117. doi:10.1007/s12609-016-0212-9
- Hill EL, Ochoa D, Denham F, et al. The Angel Wings Incision: a novel solution for mastectomy patients with increased lateral adiposity. Breast J. 2019;25(4):687-690. doi:10.1111/tbj.13301
- Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 ran-domized trial. Ann Surg. 2016;264(3):413-420. doi:10.1097/sla.0000000000001863
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. doi:10.1200/JCO.2012.45.2615
- Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor–pos-itive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):545–553. doi:10.1001/jamaoncol.2017.5524
- Hahn EE, Tang T, Lee JS, et al. Use of posttreatment imaging and biomarkers in survivors of early-stage breast cancer: inappropriate surveillance or necessary care? Cancer. 2016;122(6):908-16. doi:10.1002/cncr.29811
- Cardoso F, Kyriakides S, Ohno S, et al; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-1220. doi:10.1093/annonc/mdz173
- Jacobs C, Graham ID, Makarski J, et al. Clinical practice guidelines and consensus statements in oncology – an assessment of their methodological quality. PLoS One. 2014;9(10):e110469. doi:10.1371/journal.pone.0110469








