MOUNTAINEER-03 Propels Progress in Metastatic HER2+ CRC
The MOUNTAINEER-03 trial is designed to evaluate the efficacy of tucatinib and trastuzumab plus modified leucovorin calcium, fluorouracil, and oxaliplatin, compared with standard-of-care, first-line therapy for metastatic HER2-positive colorectal cancer.
Tanios S. Bekaii-Saab, MD

The prevalance of HER2 overexpression in metastatic colorectal cancer (CRC) is only approximately 3% to 5%. However, investigators have concluded that it presents an actionable target, and therapeutic advances with the biomarker have risen its prominence in the tumor type. Treatment strategies for this population began by mimicking successes seen in breast and gastric cancers, but investigators discovered that single-agent approaches were not as effective as in other tumor types.1
“CRC is becoming an area of significant development in the HER2 space,” Tanios S. Bekaii-Saab, MD, said in an OncLive News Network® postconference perspectives program. “HER2 is a receptor part of the HER family that essentially has HER1, HER2, HER3, HER4, and has been known to be a driver for many cancers from breast to colon to gastric, including biliary and gastroesophageal junction, [as well as] others. The story of HER2 targeting started primarily in breast, where, in addition to chemotherapy, single-agent trastuzumab [Herceptin] has moved the needle significantly for patients who overexpress HER2. From there, we saw that gastric cancer was primed for that [targeting, as well].”
Bekaii-Saab, who is leader of the Gastrointestinal Cancer Program, medical director of the Cancer Clinical Research Office, vice chair and section chief for medical oncology in the Department of Internal Medicine at Mayo Clinic Comprehensive Cancer Center in Phoenix, Arizona, noted that early data from combining single-agent trastuzumab with irinotecan suggested that there is an interesting response,2 although it did not appear to elicit the same responses observed in breast and gastric cancers. “The question became: What is the difference?
“[Early] work suggested that in colon cancer you needed dual inhibition to be able to have the maximum [effect on] CRC,” Bekaii-Saab said. The HERACLES trial (NCT03225937) conduced in Italy demonstrated that dual blockade with lapatinib (Tykerb) and trastuzumab was effective for eliciting an objective response rate (ORR) of 31% among 30 treated patients with RAS wild-type disease.3 “One of the limiting factors with lapatinib was that although it is a HER2 inhibitor, it did not have a high enough potency and it did have some HER1 inhibition. Toxicity levels were [also] a little higher.”
Following these data, Bekaii-Saab noted that other trials added to the proof of the principal that trastuzumab in combination with other inhibitors, such as pertuzumab (Perjeta) in the MyPathway study,4 showed similar outcomes in terms of response rate. “Overall, the key aspect of all of this is the patients had to have KRAS wild-type tumors to have the benefit of the dual inhibition,” he said. “An agent that was initially being looked at in breast cancer, the tyrosine kinase inhibitor tucatinib [Tukysa], which targets HER2, and is highly selective, and had minimal inhibition of EGFR, [made for] highly desirable properties in the sense that it is cleaner and more potent.”
Phase 2 Data Support Expanded Research
To evaluate the potential role of tucatinib, the phase 2 MOUNTAINEER trial (NCT03043313) was initiated. At the 2022 European Society for Medical Oncology (ESMO) World Congress on Gastrointestinal Cancer, data showed that tucatinib plus trastuzumab improved radiographic response rates in patients with metastatic HER2-positive CRC. At a median follow-up of 20.7 months, the confirmed ORR among 86 patients who received the combination was 38.1% (95% CI, 27.7%-49.3%) as assessed by blinded independent central review (BICR), with a median duration of response of 12.4 months (95% CI, 8.5-20.5). Further, the median progression-free survival (PFS) was 8.2 months (95% CI, 4.2-10.3) and the median overall survival was 24.1 months (95% CI, 20.3-36.7).5
In terms of safety, commonly reported grade 1/2 treatment-emergent adverse effects (TEAEs) among patients who received tucatinib and trastuzumab were diarrhea (60.5%), fatigue (41.9%), nausea (34.9%), and infusion-related reaction (20.9%). Grade 3 or higher TEAEs included diarrhea (3.5%) and fatigue (2.3%). In terms of overall AEs observed in the study, hypertension was the most common grade 3 or higher AE (7.0%).5
This analysis included patients who were initially enrolled in the single-arm study of the combination and those enrolled to the expansion cohort for the combination. “MOUNTAINEER was an open-label, global, phase 2 study [that] enrolled patients with RAS wild-type metastatic CRC that had progressed on prior [fluoropyrimidines], oxaliplatin, irinotecan, and anti-VEGF [monoclonal] antibodies,” John H. Strickler, MD, associate professor of medicine and a medical oncologist at the Duke University School of Medicine in Durham, North Carolina, said in an interview with OncologyLive®. “All patients enrolled had HER2-positive disease by local testing, and that could have been immunohistochemistry, [fluorescence in situ hybridization], or next-generation sequencing. These patients were initially enrolled into cohort A, which was a pilot cohort of tucatinib and trastuzumab. Based on very favorable results from that initial experience, the study was expanded. There was a randomization when the study was expanded to cohort B, which was the tucatinib/trastuzumab combination, or cohort C, which was tucatinib [alone].”
At the ESMO Congress 2022 additional data from MOUNTAINEER were presented for patients who were enrolled to an additional arm of tucatinib monotherapy and who subsequently crossed over to the combination.6 Among the 30 patients treated with tucatinib, ORR by 12 weeks was 3.3% (95% CI, 0.1%-17.2%) and disease control rate (DCR) was 80.0%. Twenty-eight patients crossed over to the combination regimen and the confirmed ORR was 17.9% (95% CI, 6.1%36.9%), with a DCR of 82.1%. Among patients who crossed over, 5 (17.9%) had a partial response, 18 (64.3%) had stable disease, and 5 (17.9%) had progressive disease.6
In cohort C, 93.3% of patients experienced any-grade AEs, including 26.7% with grade 3 or higher events. Three patients reported serious AEs. The most common AEs with tucatinib included diarrhea (33.3%), abdominal pain (20.0%), and fatigue (20.0%), all of which were grade 1 or 2. The most common grade 3 or higher AEs were increased aspartate/alanine aminotransferase (AST/ALT; 6.7% each).6
Among patients who crossed over to the combination regimen, 82.1% of patients reported any-grade AEs, with 21.4% experiencing grade 3 or higher events. Two patients had serious AEs. The most common AE was diarrhea (35.7%), of which all events were grade 1 or 2. Also in this group, the most common grade 3 or higher AEs included ALT/AST increase (7.1% and 10.7%, respectively). Two patients in the crossover group discontinued treatment because of AEs.6
“We did see [more favorable] responses after combining tucatinib with trastuzumab,” Strickler said. “For patients experiencing [AEs] and toxicity [from] chemotherapy, this is a welcome relief. Most [AEs] are manageable, and it’s fairly rare for [AEs] to lead to tucatinib discontinuation or dose reduction. [Tucatinib plus trastuzumab] represents not [only] an active regimen, but [also] a regimen that provides many patients [with] a high quality of life.”
In September 2022, the supplemental new drug application for the treatment regimen was granted priority review, with a decision date of January 19, 2023.7
“Our initial goal with MOUNTAINEER was to make tucatinib and trastuzumab an option for patients with HER2-positive metastatic CRC,” Strickler said. “[One] exciting component of [our analysis] is that we [observed] much higher response rates than expected [beyond the firstline] setting. [These] response rates [were also observed] in a tolerability profile that’s very favorable. [Accordingly, we hope] this will become a standard-of-care [SOC] option in the future for these patients.”
The next step for the combination is the ongoing, randomized, global, phase 3 MOUNTAINEER-03 trial (NCT05253651). This trial is designed to evaluate the efficacy of the combination regimen plus modified leucovorin calcium, fluorouracil, and oxaliplatin, (mFOLFOX6) compared with SOC, first-line therapy for metastatic HER2-positive CRC.8
MOUNTAINEER-03 Evaluates Dual Inhibition
SOC options for patients with HER2-positive therapy in the MOUNTAINEER-03 study include mFOLFOX6 alone, mFOLFOX6 with bevacizumab (Avastin), or mFOLFOX6 with cetuximab (Erbitux).8 Investigators will randomly assign patients with histologically and/or cytologically confirmed adenocarcinoma of the colon or rectum that is metastatic and/or unresectable to either the SOC arm or the investigative arm (Figure).8
Figure. Dual HER2 Inhibition in Metastatic CRC8
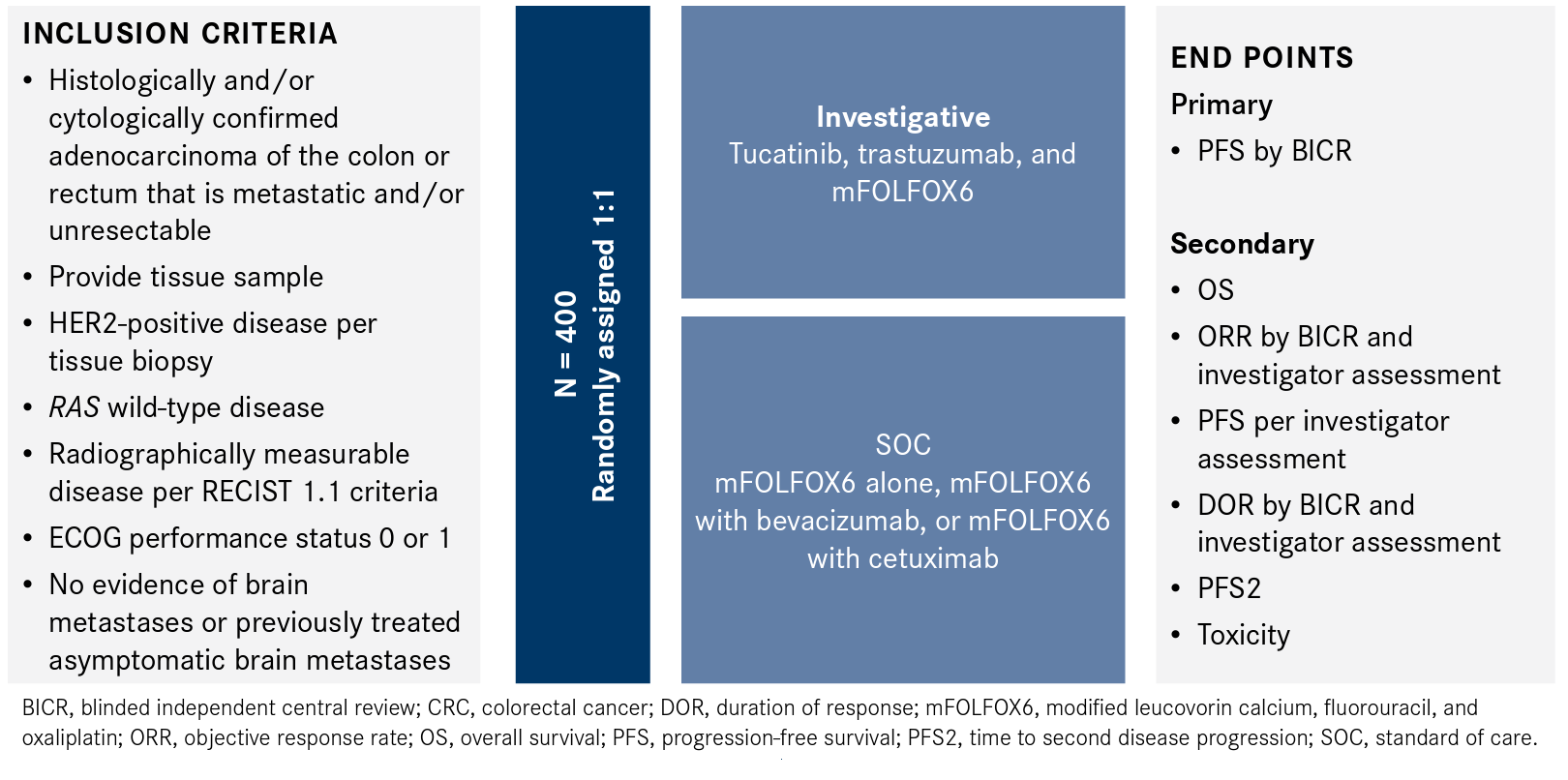
HER2-positive disease will be determined via tissue-based assay performed at a central laboratory, and patients must have RAS wild-type disease as determined by local or central testing. An ECOG performances status of 0 or 1 is also required. Patients with central nervous system requirements and those with no evidence of brain metastases or brain metastases that are asymptomatic are permitted.8
The primary end point is PFS per RECIST 1.1 criteria by BICR, with secondary outcome measures of overall survival, ORR, PFS by investigator assessment, duration of response, time to second disease progression, and toxicity. The safety analysis will include incidence of dose alterations and changes in patient-reported outcomes.8
“The key takeaway is that HER2 is an actionable target,” Strickler said. “[It] is a biomarker that we can use to select patients away from certain therapies that will be less active, [such as] antiEGFR therapies, but it’s also a biomarker we can use to select for therapies that will be more active [in some] patients….These anti-HER2 regimens [often] offer patients strong clinical benefit and high response rates. In some cases, [they even offer] long duration of response and a very favorable tolerability profile.”
MOUNTAINEER-03 is actively enrolling patients.
References
- Ahcene Djaballah S, Daniel F, Milani A, Ricagno G, Lonardi S. HER2 in colorectal cancer: the long and winding road from negative predictive factor to positive actionable target. Am Soc Clin Oncol Educ Book. 2022;42:1-14. doi:10.1200/EDBK_351354
- Ramanathan RK, Hwang JJ, Zamboni WC, et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest. 2004;22(6):858-865. doi:10.1081/cnv-200039645
- Siena S, Sartore-Bianchi A, Trusoliono L, et al. Final results of the HERACLES trial in HER2 amplified colorectal cancer. Ann Oncol. 2016;27(suppl 4):IV39. doi:10.1093/annonc/mdw335.01
- Meric-Bernstam F, Hainsworth J, Bose R, et al. MyPathway HER2 basket study: pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J Clin Oncol. 2021;39(suppl 15):3004. doi:10.1200/ JCO.2021.39.15_suppl.3004
- Seagen announces results from pivotal MOUNTAINEER trial demonstrating clinically meaningful antitumor activity of TUKYSA (tucatinib) in combination with trastuzumab in previously treated HER2-positive metastatic colorectal cancer. News release. Seagen Inc. July 2, 2022. Accessed January 5, 2023. https://bwnews.pr/3yC4xQH
- Strickler JH, Cercek A, Siena S, et al. Additional analyses of MOUNTAINEER: a phase II study of tucatinib and trastuzumab for HER2-positive mCRC. Ann Oncol. 2022;33(suppl 7):S1394. doi:10.1016/j.annonc.2022.08.023
- Seagen announces TUKYSA (tucatinib) in combination with trastuzumab granted priority review by FDA for previously treated HER2-positive metastatic colorectal cancer. News release. Seagen Inc. September 19, 2022. Accessed January 5, 2023. https://bit. ly/3DyydRB
- A study of tucatinib with trastuzumab and mFOLFOX6 versus standard of care treatment in first-line HER2+ metastatic colorectal cancer (MOUNTAINEER-03). Clinicaltrials.gov. Updated January 6, 2022. Accessed January 8, 2023. https://clinicaltrials.gov/ct2/ show/NCT05253651




