Publication
Article
New Players Join Quest for a Therapeutic Foothold Against TGFβ
Author(s):
Bintrafusp Alfa, a novel fusion protein designed to target the transforming growth factor beta pathway, racked up 3 clinical trial disappointments in less than a year, leaving the future of its development in question.
Bintrafusp Alfa, a novel fusion protein designed to target the transforming growth factor beta (TGFβ) pathway, racked up 3 clinical trial disappointments in less than a year, leaving the future of its development in question.1,2 It is one of a plethora of agents designed to target the TGFβ network that have come and gone from clinical development in the oncology field during the past 15 years.3-5
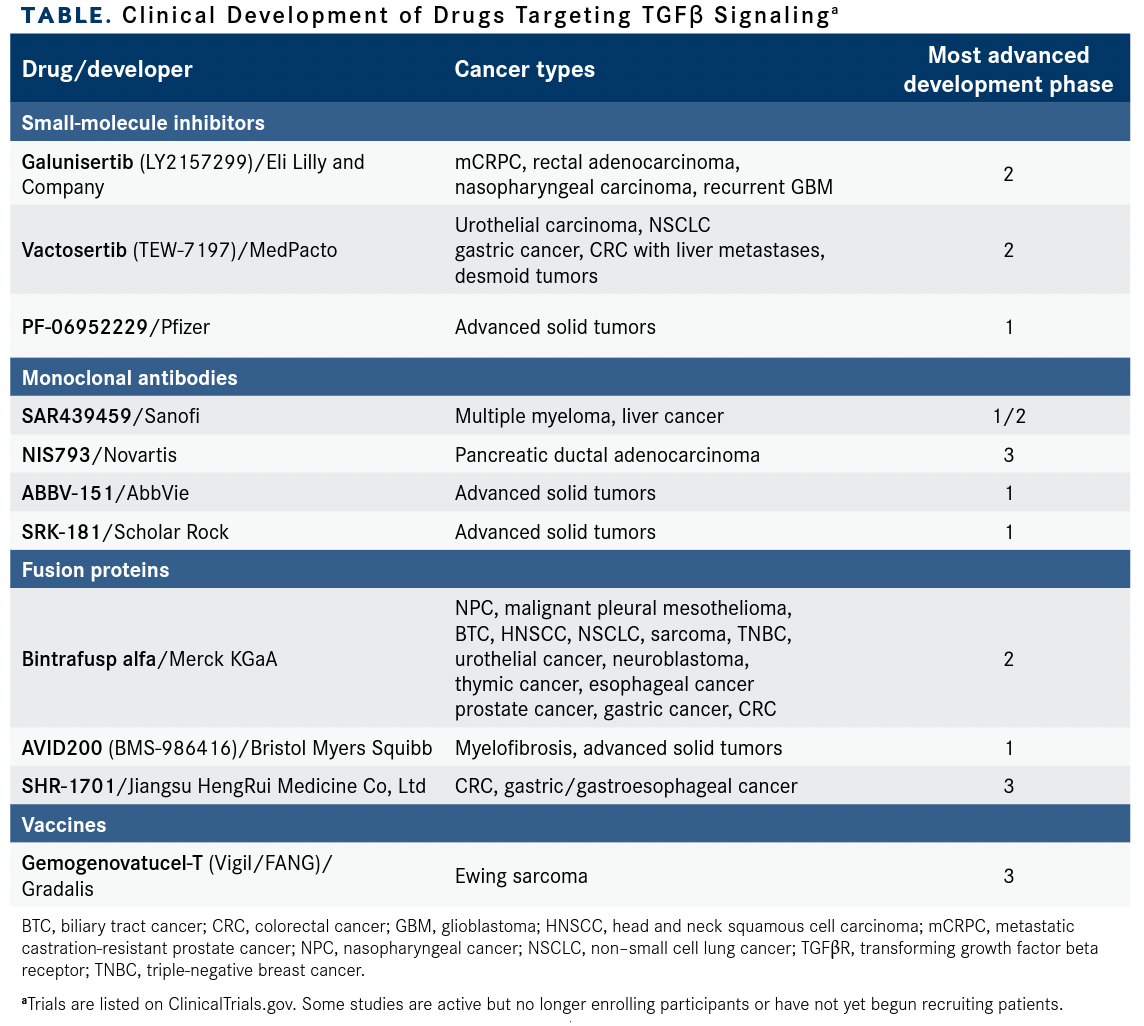
None of the TGFβ-targeting agents has gained approval for cancer therapy. However, the challenges have not stopped investigators from continuing to pursue TGFβ signaling as a therapeutic target, and novel drugs continue to enter clinical development as investigators delve more deeply into the complexities of TGFβ signaling in healthy and cancerous cells3 (TABLE).
Clinical Development of Drugs Targeting TGFβ Signalinga
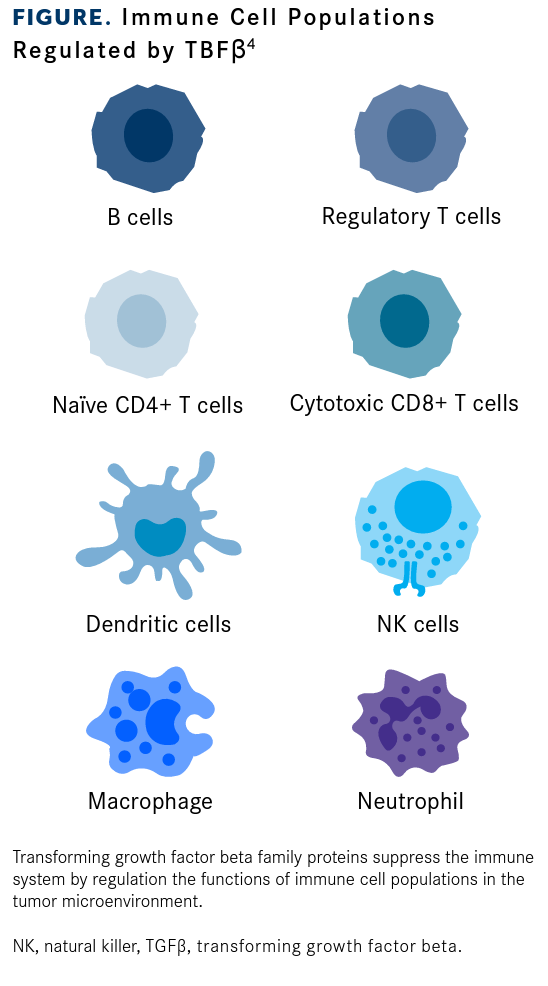
In recent years, the immunoregulatory functions of TGFβ signaling have received significant interest, particularly its potential role in resistance to immune checkpoint inhibitors (ICIs) (FIGURE4). Study findings have suggested that high levels of TGFβ in the tumor microenvironment correlate with lower response rates to ICIs and that TGFβ pathway inhibition could synergize with PD-1/PD-L1 pathway inhibition.6,7
Fusion proteins such as bintrafusp alfa, which also targets PD-L18 and is designed to block the immunosuppressive effects of TGFβ, represent just one of numerous drug designs directed at TGFβ that have been evaluated in clinical trials. Ongoing programs are evaluating monoclonal antibodies targeting the TGFβ ligands and small-molecule inhibitors of the TGFβ receptors (TGFβRs), in addition to vaccines and other strategies.3-5
Immune Cell Populations Regulated by TBFβ
Drug development has been frequently hindered by dose-limiting toxicities (DLTs) or limited clinical efficacy owing in large part to the high level of plasticity and complexity in TGFβ signaling and its apparent dual role as tumor suppressor and oncogenic activator during tumor development and progression.
Notably, TGFβ signaling plays an important role in the development of the cardiovascular system, in normal cardiac function, and in remodeling cardiac tissue after injury, and TGFβ inhibition is associated with a risk of cardiac toxicity.3,4,9 In particular, small- molecule inhibitors of TGFβRI have been shown in animal studies to be associated with dose-dependent serious cardiovascular adverse events.10,11
Small-Molecule Inhibitors
To date, the most widely studied agents targeting this pathway are 2 small-molecule TGFβRI inhibitors: galunisertib (LY2157299), developed by Eli Lilly and Company, and vactosertib (TEW-7197), developed by MedPacto. In phase 1 clinical trials, galunisertib demonstrated good tolerability and some antitumor activity in various patient populations, including patients with metastatic pancreatic cancer, glioma, and hepatocellular carcinoma (HCC).4,5,9,12 Phase 2 studies yielded mixed results; the combination of galunisertib and sorafenib (Nexavar) in patients with advanced HCC demonstrated prolonged overall survival (OS), but the combination of galunisertib and lomustine in recurrent glioblastoma did not.4,5,9,12-14 Ongoing clinical development of galunisertib is unclear; it is no longer included in the Eli Lilly pipeline,15,16 but there are several ongoing clinical trials in metastatic castration-resistant prostate cancer (NCT02452008) and rectal cancer (NCT02688712; ExIST) sponsored by the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins and Providence Health & Services, respectively, with Eli Lilly listed as collaborator.17,18
Several clinical trials have demonstrated responses and a tolerable safety profile for vactosertib in combination with PD-1/PD-L1 ICIs in patients with colorectal cancer (CRC) and non–small cell lung cancer (NSCLC) and with pomalidomide (Pomalyst) in multiple myeloma.9
Results from 2 small studies in patients with desmoid tumors and gastric adenocarcinoma were recently reported. In a phase 1/2 study (NCT03802084), 7 patients with advanced desmoid tumors were treated with 100 mg or 200 mg vactosertib twice daily on a schedule of 5 days on, 2 days off in combination with imatinib (Gleevec) 400 mg once daily. There were 2 partial responses (PRs) and 2 patients with stable disease (SD) at the 100-mg dose and 3 patients with SD at the 200-mg dose.19 In another phase 1/2 study (NCT03698825) a schedule of 5 days on, 2 days off also was applied to twice-daily vactosertib therapy (100 mg, 200 mg, or 300 mg) administered to 12 patients with gastric adenocarcinoma, who also received weekly paclitaxel (80 mg/m2). At 12 weeks, the overall response rate (ORR) was 16.7% and the disease control rate (DCR) was 83.3%.20 No DLTs or cardiac toxicity were observed in either trial.19,20
Monoclonal Antibodies
Several monoclonal antibodies directed at TGFβ are in clinical development, including pan-TGFβ antibodies that target all 3 isoforms of TGFβ (TGFβ1, 2, and 3) and others that are isoform specific. Sanofi is developing SAR439459, a fully human pan-TGFβ antibody.3,21 A phase 1 first-in-human (FIH) study (NCT03192345) testing SAR439459 as monotherapy and in combination with the PD-1 inhibitor cemiplimab-rwlc (Libtayo) is ongoing. In addition, phase 1/2 studies evaluating the agent in various combinations are underway in multiple myeloma (NCT04643002) and liver cancer (NCT04524871).
Data from the dose-escalation part of the FIH study were reported at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting. In part A, SAR439459 was administered over 6 dosing levels ranging from 0.05 to 15 mg/kg as monotherapy every 2 weeks. In part B, monotherapy dose levels cleared in dose escalation were administered in combination with a fixed dose of cemiplimab. As of January 2020, 28 patients had been enrolled to part A and 24 to part B.21
In part A, 6 patients had best response of SD, there were 2 DLTs in 8 evaluable patients at dose level 4 (3 mg/kg). In part B, 2 patients had best response of SD and 1 of 6 evaluable patients experienced DLTs with SAR439459 at dose level 5 (22 mg/kg) plus 350 mg of cemiplimab. Maximum-tolerated dose was not reached in either part. Patients continue to be enrolled to dose expansion.21
NIS793, which Novartis is developing, is another fully human pan-TGFβ antibody. Results from a phase 1b study (NCT02947165) of NIS793 as monotherapy and in combination with the PD-1 inhibitor spartalizumab (PDR001) in patients with advanced solid tumors were presented at the 2021 ASCO meeting.
Patients were initially treated with NIS793 monotherapy at doses ranging from 0.3 to 1 mg/kg every 3 weeks. Dose escalation continued in combination with spartalizumab (0.3-30 mg/kg NIS793 once every 3 weeks plus 300 mg spartalizumab once every 3 weeks or 20-30 mg/kg NIS793 once every 2 weeks plus 400 mg spartalizumab once every 4 weeks). In dose-expansion cohorts, patients with either NSCLC that was resistant to prior anti–PD-1/PD-L1 therapy (n = 20) or microsatellite-stable CRC (n = 40) were treated with 30 mg/kg NIS793 plus 300 mg spartalizumab every 3 weeks. During dose escalation of the combination, 2 patients achieved PR, 1 with clear cell renal cell carcinoma and 1 with NSCLC, while during dose expansion there were 2 PRs among patients with CRC.22
The ability of TGFβ to modulate the tumor microenvironment may be particularly important in pancreatic cancer, in which the tumor is often surrounded by dense f ibrotic stroma that can impede drug delivery. NIS793, which may reduce fibrosis by inhibiting TGFβ signaling, recently received an orphan drug designation in combination with standard chemotherapy for the treatment of patients with pancreatic cancer.4,23 The phase 2 daNIS-1 trial (NCT04390763) is evaluating the combination of NIS793 and standard chemotherapy, with or without spartalizumab, in the frontline treatment of patients with metastatic pancreatic ductal adenocarcinoma. A similar phase 3 trial (NCT04935359) in this setting, but without a spartalizumab arm, also is enrolling patients.
Meanwhile, Boston-based pharmaceutical company Scholar Rock is developing SRK-181, a monoclonal antibody designed to specifically inhibit activation of the latent (inactive precursor) form of TGFβ1, but not latent TGFβ2 or TGFβ3 or the active form of any of these proteins.24 In the recently initiated FIH DRAGON trial (NCT04291079), SRK-181 is being evaluated as monotherapy and in combination with PD-1/PD-L1 inhibitors in patients with advanced solid tumors.24 The GARP protein (also called LRRC32) binds to latent TGFβ1 on the surface of regulatory T cells and platelets, and this complex regulates the bioavailability and secretion of TGFβ1. Another novel antibody, AbbVie’s ABBV-151, binds to the GARP-latent TGFβ1 complex and inhibits TGFβ1 release from the cell surface.25-27
Therapeutic Vaccines
Several therapeutic cancer vaccines designed to prevent TGFβ synthesis and activation have been developed but clinical trials have yielded mixed results. NovaRx Corporation developed belagenpumatucel-L (Lucanix), a cancer vaccine composed of 4 irradiated human NSCLC cell lines transfected with a TGFβ2 antisense gene. The vaccine showed promise in patients with advanced NSCLC; however, in a phase 3 trial (NCT00676507) of belagenpumatucel-L compared with placebo as maintenance therapy for patients with advanced NSCLC treated with platinum-based chemotherapy, there was no significant difference in OS.3,4,9,12
Gemogenovatucel-T (Vigil; formerly known as FANG) is an autologous vaccine in which the tumor cells are transfected with a plasmid containing the granulocyte-macrophage colony-stimulating factor (GM-CSF, also called CSF2) gene and a short hairpin RNA targeting furin. The therapy, which Gradalis, Inc is developing, is designed to reduce the protein expression of furin, a protease involved in the processing of latent TGFβ1 and TGFβ2 into the active form.12,28,29
Investigators have carried out phase 2 clinical trials in several cancer types, including melanoma (NCT01453361) and CRC (NCT01505166), that were terminated for business reasons.26,28 Clinical development is ongoing, however, and results of the phase 2b VITAL trial (NCT02346747), in which gemogenovatucel-T was evaluated as frontline maintenance therapy in patients with ovarian cancer, were recently published.30
In the overall study population, median recurrence-free survival (RFS) was higher with the vaccine at 11.5 months (95% CI, 7.5-not reached) vs 8.4 months (95% CI, 7.9-15.5) with placebo but was not statistically significant (HR, 0.688; 90% CI, 0.44-1.07; P = .078). However, the vaccine showed greater efficacy in patients with wild-type BRCA1/2 gene status (HR, 0.51; 90% CI, 0.30-0.88; P = .02).30 In the homologous recombination–proficient patients, median RFS was 10.6 months (95% CI, 5.9-not available) in the Vigil group vs 5.7 months (95% CI, 5.6-14.9) in the placebo group (HR, 0.386; 90% CI, 0.199-0.750; P = .007), according to a subgroup analysis presented at the 2021 ASCO meeting.31
Data from phase 1 and 2 clinical trials suggest that gemogenovatucel-T may have activity in combination with irinotecan and temozolomide in the treatment of patients with Ewing sarcoma.32,33 These data provided the rationale for the phase 3 VITA trial (NCT03495921) of this combination in patients with metastatic Ewing sarcoma with relapsed/refractory disease after 1 prior line of chemotherapy. This trial is ongoing but not actively recruiting participants, according to ClinicalTrials.gov.34
Fusion Proteins
In January 2021, bintrafusp alfa was one of the most notable TGFβ pathway–targeting agents in development. The fusion protein is composed of an antibody targeting PD-L1 fused to the extracellular domains of 2 TGFβRII molecules.3
Bintrafusp alfa has been evaluated in several tumor types; the agent elicited ORRs of approximately 20% across several studies in patients with advanced NSCLC and esophageal, gastric, and biliary tract cancers (BTCs).8,35-37 In patients with NSCLC, response rates were higher among patients with PD-L1positive tumors (PD-L1 expressed by ≥ 1% of tumor cells; ORR, 36.0%), especially those with high PD-L1 levels (PD-L1 expressed by ≥ 80% of tumor cells; ORR, 85.7%).8
These data prompted progression into phase 2 and 3 clinical trials in NSCLC and BTC, but the results of these trials ultimately proved to be a source of significant disappointment for Merck KGaA and GlaxoSmithKline, the companies developing the drug. In January 2021, a review of data from the phase 3 INTR@PID Lung 037 trial (NCT03631706) in f irst-line treatment of patients with NSCLC with high PD-L1 expression levels prompted the companies to discontinue the trial based on the recommendation of the independent data monitoring committee (IDMC), which felt that it was unlikely that the trial would meet the coprimary end point of improved progression-free survival.38
Then, in March 2021, topline data from the phase 2 INTR@PID BTC 047 trial (NCT03833661), which examined the safety and efficacy of second-line treatment with 1200 mg bintrafusp alfa administered once every 2 weeks to 159 patients with BTC, did not meet the predefined threshold for regulatory filing in this setting.39 Finally, in August 2021, Merck announced that it was discontinuing the phase 2/3 INTR@PID BTC 055 trial (NCT04066491) based on a review of the data conducted by the IDMC, which concluded the study was unlikely to meet its primary objective of improved OS.1
A month later, the 2 companies announced that they were ending their partnership involving bintrafusp alfa, a deal that once was valued at $4.2 billion, effective September 30.1,2 Merck, which retains control of the drug, said the company would use advanced analytics to examine the trove of clinical data generated by studies into the drug. “The important insights this program has yielded about the biology of TGFβ will inform the collective understanding of this pathway,” Merck said in a statement.40 Nearly 40 clinical trials evaluating bintrafusp alfa are ongoing or actively recruiting patients, including studies in cervical, breast, and prostate cancers, and NSCLC, according to ClinicalTrials.gov.
A second TGFβ–PD-L1 bifunctional fusion protein, SHR-1701, is being developed by a Chinese company, Jiangsu HengRui Medicine Co, Ltd. Preliminary results from a phase 1 trial (NCT03774979) in 24 patients with advanced NSCLC harboring EGFR mutations demonstrated an ORR of 16.7% (95% CI, 4.7%-37.4%) and a DCR of 50.0% (95% CI, 29.1%-70.9%) among patients treated with SHR-1701 at a dose of 3, 10, or 20 mg/kg every 3 weeks or 20 mg/kg every 2 weeks.41
AVID200, a fusion protein comprising TGFβR extracellular domains fused to a human Fc domain, also is in development. This “TGFβ ligand trap” selectively binds and neutralizes TGFβ1 and TGFβ3, enhancing antitumor immunity. It is hoped that not blocking TGFβ2 will reduce the risk of cardiovascular toxicity.9,42
References
- Armstrong A. Merck KGaA’s $4.2B bintrafusp alfa nabs a 3rd strike. Is the GSK-partnered cancer hopeful out? FierceBiotech. August 23, 2021. Accessed September 28, 2021. https://www.fiercebiotech.com/biotech/merck-s-4-2b-bintrafusp-alfa-nabs-a-3rd-strike-cancer-hopeful-out
- Armstrong A. GSK’s $4.2B bet on Merck KGaA's cancer drug goes up in smoke as pharmas cut ties after series of flops. FierceBiotech. September 30, 2021. Accessed October 26, 2021. https://www.fiercebiotech.com/biotech/merck-kgaa-gsk-cut-ties-bintrafusp-alfa-deal-had-4-2b-biobucks-line
- Ciardiello D, Elez E, Tabernero J, Seoane J. Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Ann Oncol. 2020;31(10):1336-1349. doi:10.1016/j.annonc.2020.07.009
- Liu S, Ren J, Ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):8. doi:10.1038/s41392-020-00436-9
- Yang Y, Ye WL, Zhang RN, et al. The role of TGF-β signaling pathways in cancer and its potential as a therapeutic target. Evid Based Complement Alternat Med. 2021;2021:6675208. doi:10.1155/2021/6675208
- Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544-548. doi:10.1038/nature25501
- Tauriello DVF, Palomo-Ponce S, Stork D, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538-543. doi:10.1038/nature25492
- Paz-Ares L, Kim TM, Vicente D, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. 2020;15(7):1210-1222. doi:10.1016/j.jtho.2020.03.003
- Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol. 2021;14(1):55. doi:10.1186/s13045-021-01053-x
- Anderton MJ, Mellor HR, Bell A, et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39(6):916-924. doi:10.1177/0192623311416259
- Stauber AJ, Credille KM, Truex LL, Ehlhardt WJ, Young JK. Nonclinical safety evaluation of a transforming growth factor β receptor I kinase inhibitor in Fischer 344 rats and beagle dogs. J Clin Pract. 2014;4(3):1000196. doi:10.4172/2161-0495.196
- Huang CY, Chung CL, Hu TH, Chen JJ, Liu PF, Chen CL. Recent progress in TGF-β inhibitors for cancer therapy. Biomed Pharmacother. 2021;134:111046. doi:10.1016/j.biopha.2020.111046
- Brandes AA, Carpentier AF, Kesari S, et al. A phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016;18(8):1146-1156. doi:10.1093/neuonc/now009
- Kelley RK, Gane E, Assenat E, et al. A phase 2 study of galunisertib (TGF-β1 receptor type I inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clin Transl Gastroenterol. 2019;10(7):e00056. doi:10.14309/ctg.0000000000000056
- Medicines in development. Lilly. Updated October 25, 2021. Accessed September 23, 2021. https://www.lilly.com/discovery/clinical-development-pipeline
- Adams B. Eli Lilly cuts 3 cancer drugs amid Q4 clear-out. FierceBiotech. January 30, 2020. Accessed September 28, 2021. https://www.fiercebiotech.com/biotech/eli-lilly-cuts-three-cancer-drugs-amid-q4-clear-out
- Study of TGF-β receptor inhibitor galunisertib (LY2157299) and enzalutamide in metastatic castration-resistant prostate cancer. ClinicalTrials.gov. Updated October 1, 2021. Accessed September 28, 2021. https://clinicaltrials.gov/ct2/show/NCT02452008
- ExIST study of LY2157299 (Galunisertib) in rectal cancer. ClinicalTrials.gov. Updated September 16, 2020. Accessed September 30, 2021. https://clinicaltrials.gov/ct2/show/NCT02688712
- Kim HS, Ahn JH, Kim JE, et al. A phase I study of TGF-β inhibitor, vactosertib in combination with imatinib in patients with advanced desmoid tumor (aggressive fibromatosis). J Clin Oncol. 2020;38(suppl 15):11557. doi:10.1200/JCO.2020.38.15_suppl.11557
- Jung M, Lee CK, Kim HS, et al. 1453P safety and efficacy of vactosertib, a TGF-βR1 kinase inhibitor, in combination with paclitaxel in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2020;31(suppl 4):S912. doi:10.1016/j.annonc.2020.08.1959
- Williamson SK, Hodi FS, Johnson ML, et al. Safety, pharmacokinetic and pharmacodynamic results from dose escalation of SAR439459, a TGFβ inhibitor, as monotherapy or in combination with cemiplimab in a phase 1/1b study. J Clin Oncol. 2021;39(suppl 15):2510. doi:10.1200/JCO.2021.39.15_suppl.2510
- Bauer TM, Lin CC, Greil R, et al. Phase Ib study of the anti-TGF-β monoclonal antibody (mAb) NIS793 combined with spartalizumab (PDR001), a PD-1 inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2021;39(suppl 15):2509. doi:10.1200/JCO.2021.39.15_suppl.2509
- Novartis receives FDA orphan drug designation for NIS793 in pancreatic cancer. Novartis. July 27, 2021. Accessed September 28, 2021. https://www.novartis.com/news/novartis-receives-fda-orphan-drug-designation-nis793-pancreatic-cancer
- Yap TA, Barve MA, Gainor JF, et al. First-in-human phase 1 trial (DRAGON) of SRK-181, a potential first-in-class selective latent TGFβ1 inhibitor, alone or in combination with anti-PD-(L)1 treatment in patients with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl):TPS3146. doi:10.1200/JCO.2021.39.15_suppl.TPS3146
- ABBV-151. AbbVie. Accessed September 28, 2021.
https://www.abbvie.com/our-science/pipeline/abbv-151.html - Wang R, Zhu J, Dong X, Shi M, Lu C, Springer TA. GARP regulates the bioavailability and activation of TGFβ. Mol Biol Cell. 2012;23(6):1129-1139. doi:10.1091/mbc.E11-12-1018
- Guo M, Wu W, Liu W, Ren F. Mini-review: GARP, a putative potential molecule in tumor immunosuppressive environment. J Cancer Treatment Diagn. 2019;3(1). doi:10.29245/2578-2967/2019/1.1164
- Gómez-Gil V. Therapeutic implications of TGFβ in cancer treatment: a systematic review. Cancers (Basel). 2021;13(3):379. doi:10.3390/cancers13030379
- Rocconi RP, Stanbery L, Madeira da Silva L, et al. Long-term follow-up of gemogenovatucel-T (Vigil) survival and molecular signals of immune response in recurrent ovarian cancer. Vaccines (Basel). 2021;9(8):894. doi:10.3390/vaccines9080894
- Rocconi RP, Grosen EA, Ghamande SA, et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020;21(12):1661-1672. doi:10.1016/S1470-2045(20)30533-7
- Rocconi RP, Ghamande SA, Barve MA, et al; The Vigil Team. Maintenance vigil immunotherapy in newly diagnosed advanced ovarian cancer: efficacy assessment of homologous recombination proficient (HRP) patients in the phase IIb VITAL trial. J Clin Oncol. 2021;39(suppl 15):5502. doi:10.1200/JCO.2021.39.15_suppl.5502
- Gradalis presents initial data from phase II U.S. trial for Ewing’s sarcoma at the annual meeting of the Children’s Oncology Group. News release. Gradalis, Inc. November 9, 2018. Accessed September 27, 2021. https://apnews.com/press-release/Globe%20Newswire/eb005397970e7188ab333d074e2346f2
- Ghisoli M, Barve M, Mennel R, et al. Three-year follow up of GMCSF/bi-shRNA(furin) DNA-transfected autologous tumor immunotherapy (Vigil) in metastatic advanced Ewing’s sarcoma. Mol Ther. 2016;24(8):1478-1483. doi:10.1038/mt.2016.86
- Vigil + irinotecan and temozolomide in Ewing’s sarcoma (VITA). ClinicalTrials.gov. Updated August 18, 2021. Accessed September 27, 2021. https://clinicaltrials.gov/ct2/show/NCT03495921
- Tan B, Khattak A, Felip E, et al. M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with post-platinum esophageal adenocarcinoma (EAC): preliminary results from a phase I cohort. Ann Oncol. 2018;29(suppl 8):VIII216. doi:10.1093/annonc/mdy282.027
- Yoo C, Oh DY, Choi HJ, et al. M7824 (MSB0011359C), a bifunctional fusion protein targeting transforming growth factor β (TGF-β) and PD-L1, in Asian patients with pretreated biliary tract cancer (BTC): efficacy by BTC subtype. Ann Oncol. 2018;29(suppl 9):IX48-IX49. doi:10.1093/annonc/mdy432.005
- Bang YJ, Doi T, Kondo S, et al. Updated results from a phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with pretreated recurrent or refractory gastric cancer. Ann Oncol. 2018;29(suppl 8):VIII222-VIII223. doi:10.1093/annonc/mdy282.045
- Merck KGaA, Darmstadt, Germany announces update on the INTR@PID clinical program including Lung 037 study. News release. Merck KGaA. January 20, 2021. Accessed September 24, 2021. https://www.prnewswire.com/news-releases/merck-kgaa-darmstadt-germany-announces-update-on-the-intrpid-clinical-program-including-lung-037-study-301211771.html
- Merck KGaA, Darmstadt, Germany, reports topline data for bintrafusp alfa as second-line monotherapy treatment in biliary tract cancer. News release. Merck KGa. March 16, 2021. Accessed September 24, 2021. https://www.emdgroup.com/en/news/bintrafusp-topline-data-biliary-tract-cancer-16-03-2021.html
- Merck announces mutual decision to end bintrafusp alfa agreement with GSK. News release. Merck. September 30, 2021. Accessed October 27, 2021. https://www.businesswire.com/news/home/20210930005596/en/Merck-Announces-Mutual-Decision-to-End-Bintrafusp-Alfa-Agreement-With-GSK
- Shi M, Chen J, Li KY, et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, for advanced NSCLC with EGFR mutations: data from a multicenter phase 1 study. J Clin Oncol. 2021;39(suppl 15):9055. doi:10.1200/JCO.2021.39.15_suppl.9055
- TGFbeta receptor ectodomain-IgG Fc fusion protein BMS-986416 (code C159817). NCI Thesaurus. Accessed September 28, 2021. https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&version=20.02d&code=C159817&ns=ncit







