Patient Outcomes Support Cabazitaxel Over Standard in mCRPC
Findings from a preplanned analysis of the phase 4 CARD study demonstrated superior patient-reported outcomes with cabazitaxel in men with castration-resistant prostate cancer who received prior docetaxel and androgen receptor targeted therapy versus alternative AR-targeted treatment options.
Findings from a preplanned analysis of the phase 4 CARD study (NCT02485691) demonstrated superior patient-reported outcomes (PROs) with cabazitaxel (Jevtana) in men with castration-resistant prostate cancer (mCRPC) who received prior docetaxel and androgen receptor (AR)targeted therapy versus alternative AR-targeted treatment options.
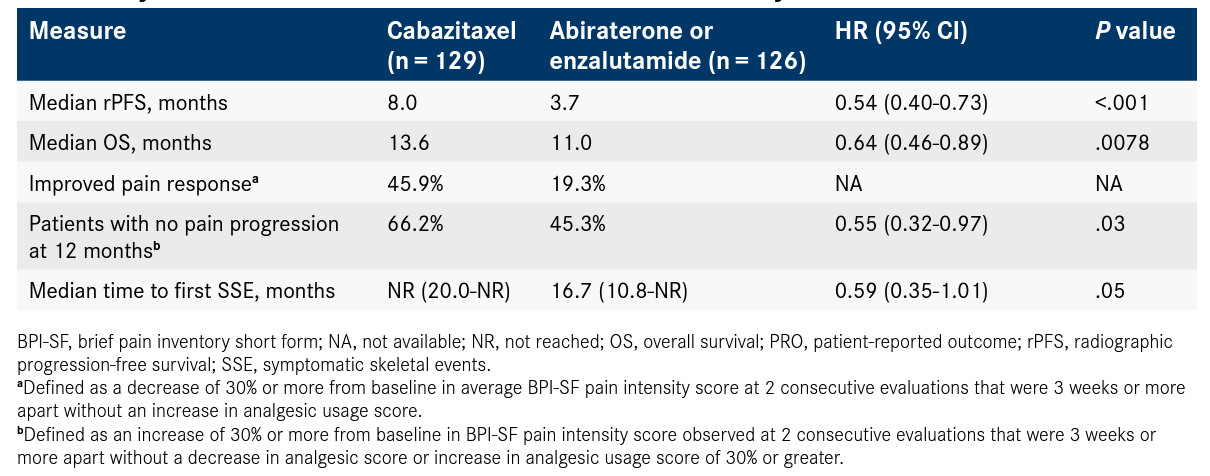
Data presented at the 2020 American Urological Association (AUA) Annual Meeting showed that cabazitaxel improved pain response compared with abiraterone acetate (Zytiga) or enzalutamide (Xtandi) in 45.9% and 19.3% of patients, respectively (P <.0001). Patients on cabazitaxel had longer time to pain progression (HR, 0.55; 95% CI, 0.32-0.97; P = .03) compared with those treated with AR-targeted agents.
The time to symptomatic skeletal events was also significantly delayed with cabazitaxel compared with AR-targeted therapy (HR, 0.59; 95% CI, 0.35-1.01; P = .05; Table1,2). These events have a large impact on quality of life for patients with mCRPC, according to Gero Kramer, MD, who presented the findings during the virtual meeting.
Key Outcomes Measures and PROs From the CARD Study1,2
Cabazitaxel had a manageable safety profile, as well as fewer cardiac disorders compared with the control arm (4.8% vs 0.8%, respectively). Only 3.2% of patients given cabazitaxel had febrile neutropenia due to prophylactic granulocyte-colony stimulating factor given for each cycle.
Data regarding PROs were measured using the 5-level EuroQol Group’s 5-Dimension (EQ-5D-5L) questionnaire. Patient scores were evaluated at baseline, on the first day of each cycle (every 3 weeks), and at the final treatment visit. Patients were required to receive at least 1 dose of cabazitaxel or AR-targeted therapy, have an EQ-5D-5L assessment at baseline, and complete a minimum of 1 subsequent evaluation to be eligible to participate. Investigators also performed a mixed-effect model repeated measures analysis of EQ-5D-5L changes from baseline.
The 5 health domains assessed by the generic house status utility instrument EQ-5D-5L are mobility, self-care, usual activities, pain/discomfort, and anxiety/ depression. These domains are rated on a Likert-type scale, with 5 options: no problems, slight problems, moderate problems, severe problems, and extreme problems. Visual analog scale (VAS) is rated from 0 to 100 for overall health.
Changes in utility index scores and VAS from baseline both during treatment and at the end of treatment (EOT) favored cabazitaxel. The least squares mean difference ranged from 0.04 to 0.08 (0.05 at EOT) for utility index and 1.6 to 6.4 (5.9 at EOT) for VAS between cabazitaxel and AR-targeted therapy. Both groups had a 0.70 mean utility score at baseline and a comparable VAS (65.8, cabazitaxel; 66.3, AR-targeted therapy).
Among the patients treated with cabazitaxel, 45 (39.1%) reported moderate to severe or extreme pain/discomfort evaluated by EQ-5D-5L at baseline; 47 (40.9%) reported the same with AR-targeted therapy.
“Changes in VAS and utility score of EQ-5D-5L numerically favored cabazitaxel,” Kramer, an associate professor of urology in the Department of Urology at the Medical University of Vienna, in Austria, said of the data. “Results support the use of cabazitaxel over abiraterone or enzalutamide as a standard of care in patients previously treated with docetaxel who progressed within 12 months with alternative AR-targeted therapy.”
The CARD trial enrolled 255 patients, 230 of whom were evaluable for EQ-5D-5L.1 In previously reported results of CARD, cabazitaxel significantly improved median radiographic progression-free survival (PFS) by more than 4 months (8.0 months vs 3.7 months; HR, 0.54; 95% CI, 0.40-0.73; P <.001). Median overall survival with cabazitaxel was 13.6 months versus 11.0 months for patients receiving abiraterone or enzalutamide (HR, 0.64; 95% CI, 0.46-0.89; P = .0078).2
CARD was a multicenter, randomized, open-label study of patients with mCRPC who had progressed within 12 months of receiving AR-targeted therapy before or after docetaxel. Patients were randomized 1:1 to cabazitaxel every 3 weeks plus prophylactic granulocyte colony-stimulating factor or abiraterone or enzalutamide once daily. The median follow-up was 9.2 months, and the primary end point was radiographic PFS. Secondary end points included overall survival, PFS, prostate-specific antigen response, and tumor response.
Referencess
- Kramer G, Sternberg CN, Fizazi K, et al. Effect of cabazitaxel vs abiraterone or enzalutamide on patient-reported outcomes in metastatic castration-resistant prostate cancer: a pre-planned EQ-5D-5L analysis of the CARD study. J Urol. 2020;203(suppl 4; abstr PD16-08):e366. doi:10.1097/JU.0000000000000859.08
- de Wit R, de Bono J, Sternberg CN, et al; CARD Investigators. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506‐2518. doi:10.1056/NEJMoa1911206




