Publication
Article
Pazdur Followed the Pathway of Greatest Resistance to the FDA
Author(s):
Richard Pazdur, MD, set out to be a leader in medicine and a teacher of doctors. He achieved that and more, turning the oncology drugs section of the FDA from a poorly understood and lead-footed division into a fast-moving and patient-responsive entity.
Richard Pazdur, MD
Richard Pazdur, MD, a 2019 winner of the OncLive® Giants of Cancer Care® award for community outreach, education, and cancer policy, set out to be a leader in medicine and a teacher of doctors. He achieved that and more, turning the oncology drugs section of the FDA from a poorly understood and lead-footed division into a fast-moving and patient-responsive entity.
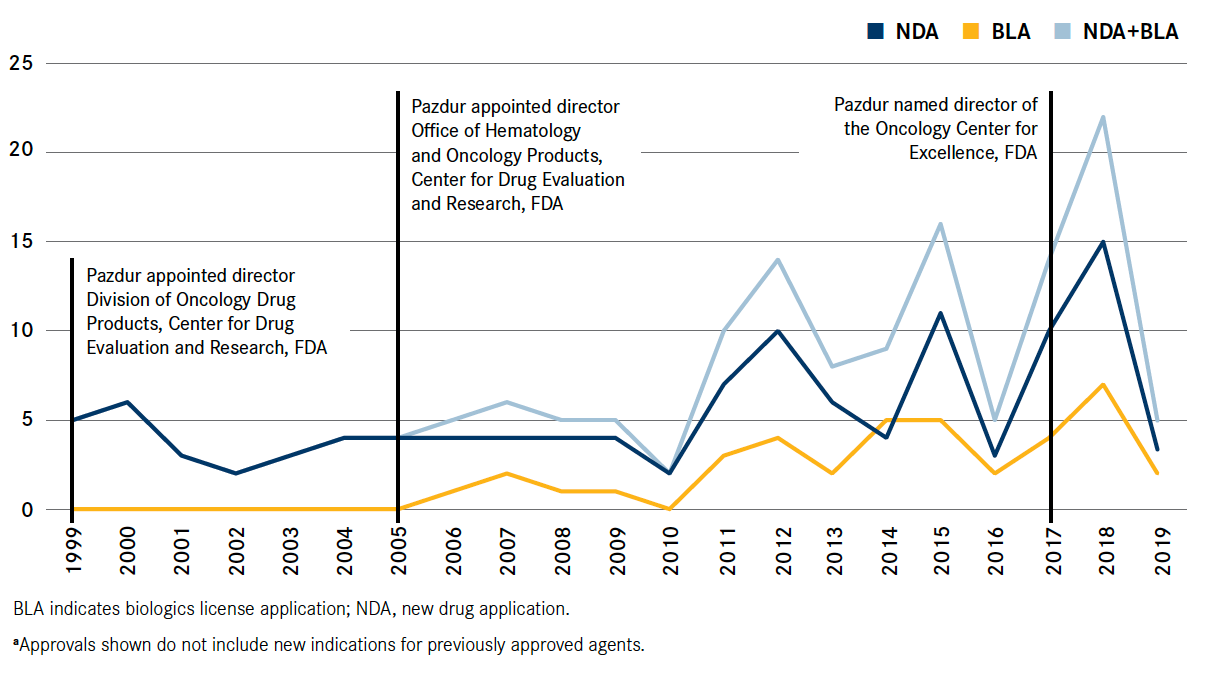
Pazdur, now director of the FDA’s Oncology Center of Excellence (OCE), introduced reforms and broadened operations that enabled the agency to keep pace with a breathtaking evolution of oncology drugs. In 1979, just 35 oncology agents were available, predominantly chemotherapies with limited efficacy and high toxicity. Today, the FDA approves many new molecular agents each year, and many of these agents are leading to better response rates with lower toxicity than past treatments (Figure1).
Under Pazdur, the FDA has implemented congressional mandates to consider new end points beyond overall survival and unshackled the review process from the cumbersome requirements of randomized trials, which are often impractical in this era of targeted medicine. Often, Pazdur said, novel agents are so obviously superior to standard of care that no patient would participate in a randomized trial knowing that somebody else might get the experimental drug.
FIGURE. New Molecular Entities Approved in Oncology/Hematology From 1999 to Present
New Molecular Entities Approved in Oncology/Hematology From 1999 to Present
“When we make a decision about approving a drug, it has to be patient centered. It can’t be about the regulations,” Pazdur said, describing the philosophy he has championed at the FDA. It did not always seem that way to a skeptical public. Pazdur was once publicly vilified as “Dr Death” for his conservative approach. The Wall Street Journal was particularly critical, publishing a series of editorials that criticized Pazdur for what they felt was an overly conservative approach. “I believe I had my own editorial writer at the Wall Street Journal,” he said. A 2002 editorial headline in the paper read “FDA to Patients: Drop Dead”; and a 2005 editorial was titled “Pazdur’s Cancer Rules.”2,3
Pazdur said he was always as strong a patient advocate as anybody, but experimental drugs had to get the scrutiny they deserved. “I think that many of the drugs that we did not approve just generally should not have been approved. They were classical chemotherapy drugs that had a lot of toxicity and very little evidence of efficacy,” he said.
Public attitudes were fierce at times, and on occasion Pazdur went to public meetings accompanied by a bodyguard, but the support of a tight and professional staff at the FDA lent confidence amid the clamor for better drugs. The other side of confidence is drawing strength from having done the homework, he said. Decisions do not occur in a vacuum. Application materials have crossed his desk multiple times before a decision whether to approve is made. “We’re discussing these applications for many, many months while they’re being reviewed, and we’re having 2 meetings every week where we discuss important applications, and we have the opinions of many oncologists here as well as on the outside who give us perspectives on the issues with applications. When we go to an [Oncology Drugs Advisory Committee] meeting, it is a very rare situation that I encounter where I hear something entirely new that we haven’t heard already in the agency about an application,” he said.
“One of the things you have to realize in this job is that not everybody is going to agree with you, that’s for sure. When you’re approving drugs, either you’re approving them too slowly or you’re not approving the ones that should be approved,” Pazdur said.
A Leader From the Start
Pazdur grew up in Calumet City, Illinois, a working-class community southeast of Chicago that is the famed fictitious birthplace of Jake and Elwood Blues, from the movie The Blues Brothers. His father was blind, and as a young man, Pazdur had a huge share of family responsibility, which included working part-time jobs to help make ends meet. “It had a profound influence on me financially because, obviously, my parents looked to me for support. I had a lot of responsibilities,” he shared.
Despite these duties, Pazdur excelled at his schoolwork, completing high school, college, and medical school each in 3 years. He graduated from Northwestern University with highest distinction.
As early as his sophomore year in high school, Pazdur knew he wanted to be a doctor, but not just any doctor. He wanted to be a teacher of doctors.
“I really had a clear idea that I wanted to go into academic medicine,” he noted. That said, it took a while for him to decide on a medical focus. He took rotations in psychiatry and internal medicine, but he could not see himself in either discipline for the rest of his life. His final rotation was in oncology, and it was a winner.
“Unlike other areas of internal medicine, oncology was really a total picture of the human body,” Pazdur said. “I also was very interested in the area of clinical pharmacology— toxicities and how drugs interacted with one another.”
Oncology programs of 40 years ago, when Pazdur earned his MD, were not very extensive. He did his fellowship at Rush University Medical Center of Chicago, which, despite having the largest oncology program of its type in the city, employed only 10 to 12 medical oncologists.
Pazdur’s outstanding qualities as a doctor and leader in medicine were evident when he started his fellowship, said Philip Bonomi, MD, a thoracic oncologist and the Alice Pirie Wirtz Professor of Medical Oncology at Rush. Bonomi employed a young oncology nurse named Mary Bagby who would go on to marry Pazdur.
Bonomi became a teacher and mentor to Pazdur. He said that even as a young doctor, Pazdur was incredibly bright, a very careful thinker, and “above all, very compassionate.”
He added that the profound changes at the FDA under Pazdur’s watch serve as evidence of Pazdur’s dedication and commitment.
“Many new drugs have been approved very rapidly, and that has happened under his leadership. He has streamlined the way this works,” Bonomi said.
Pazdur’s excellent rapport with others also contributes to his success, Bonomi added. “They like him, and he likes them. That’s what it boils down to. He’s a level-5 leader in my opinion: passionate about getting people involved and passionate about doing the right thing, and the right thing is about bringing safe and effective drugs to as many people as possible,” Bonomi said.
Pazdur later applied for and was appointed to a position at Wayne State University School of Medicine in Detroit, Michigan. Wayne State had a very large program—roughly 40 medical oncologists devoted to various specific tumor types—plus phase I and II contracts with the National Cancer Institute. “At that time in Chicago in the 1970s, there wasn’t any place that even remotely resembled what was going on at Wayne State,” so he accepted a faculty job there, Pazdur said. He had to break the news to his new bride that they were going to spend their honeymoon in Detroit searching for housing.
“I said we were moving to Detroit, and she said, ‘Rick, you don’t even know where Detroit is!’” he said.
It was a fabulous experience, he recounted, noting the opportunity to direct the oncology fellowship program and develop his skills in training people. “I worked on phase I drug development, gastrointestinal cancer, and predominantly lung cancer.”
Into the “Black Box”
In 1988, Pazdur moved on to head the Gastrointestinal Department at The University of Texas MD Anderson Cancer Center in Houston, where he worked with Bernard Levin, MD. In addition to training fellows, he conducted drug development work and gained experience going before FDA panels with pharmaceutical company representatives to discuss drug applications.
At the time, important events were taking shape that were not well understood and which Pazdur wouldn’t have predicted. For one, he thought that clinical trials would always be run by large medical centers, especially in oncology, and that drugs would be developed through the National Cancer Institute. He thought that pharma would instinctively turn away from oncology drug development because efficacies were so poor and toxicities were so high. On these points, “I couldn’t have been more wrong,” Pazdur said. Nowadays, he notes, cancer drugs amount to 40% of all drug sales.
Over time, he began to understand the FDA more clearly. He had always regarded the agency as a sort of “black box,” an obscure entity whose innermost workings were known to only a select few. “Nobody goes to medical school—nobody puts on their medical school application that they want to work for the FDA,” he said.
“I really saw at that time, relatively late in my career, the importance of the FDA and the influence they had not only on clinical trials but on what drugs were developed and how we view safety.”
In 1999, Pazdur applied for a job as director of the Division of Oncology Drug Products at the FDA. He did not think it was going to be a long interview and was surprised when he got the job. He saw a great deal of opportunity to put his organizational and staff development skills to work. “There was a tremendous need to expedite drug development to get agents to patients who had few options, and that was kind of heralded by the accelerated drug approval regulations being approved at the time in the late 1990s,” he said.
Helping the transformation along was the FDA Modernization Act of 1997, which expedited the fast-track approvals process for drugs and biologics intended for serious or life-threatening conditions. That was followed in 2012 by passage of the FDA Safety Innovations Act, which allowed for accelerated approval based on surrogate or intermediate clinical end points.
The oncology drugs section was only lightly staffed when Pazdur came aboard. It included just 10 medical oncologists, and they did not specialize in disease types. Drawing from his experience at the University of Chicago, he had each oncologist focus on particular cancers and built up the staff from there. Today, more than 100 medical oncologists work on drug approvals at the FDA.
“We’ve really remodeled the divisions after the major cancer centers that have teams to work on specific diseases,” Pazdur said. “One of the things I tried to emphasize here was a more transparent process of how we develop drugs and really a much more academic approach to drug development and drug evaluation.”
In addition to heading the OCE, created 2 years ago through the National Cancer Institute’s Cancer Moonshot initiative, Pazdur remains acting director of the Office of Oncologic Diseases—formerly the Office of Hematology and Oncology Products—within the FDA’s Center for Drug Evaluation and Research.
The FDA has come a long way over the years, even though the public has not always been appreciative of its efforts. Pazdur once noted in a public address that “I felt like crawling under my seat” when he saw the 2013 movie Dallas Buyers Club, which depicted the FDA as a hard-nosed bureaucracy that made it unnecessarily difficult for patients, especially those with AIDS, to obtain the drugs they needed.4 In the biographical movie, a man infected with AIDS crosses the border to Mexico to obtain drugs that are effective against the disease, then begins selling them to people suffering from AIDS in Texas.
It helped that drugs got better, making it easier for the FDA to approve them. Still, some have speculated that Pazdur was not moved to make any reforms until after Mary received a diagnosis of ovarian cancer in 2012. In fact, Pazdur said, the faster pace of approvals began for different reasons. “It has nothing to do with a change in me. Our drugs are just much better because they’re targeted.”
Pazdur said he was wrongly characterized in a 2016 New York Times article that said he was so shaken by his wife’s death that he unleashed a wave of approvals of risky new drugs. “No, that did not happen. I want to make that clear,” Pazdur said. “I think it had an influence on my personal thinking with regard to drug toxicities and, actually, the need to provide safe and effective drugs. My wife’s death did not lead to any change of standards or anything like that.”
His wife’s ordeal with treatment-related adverse events was the true eye-opener. “One can read all about drug toxicities, but until you experience them personally or through a family member, they have a different meaning,” he said.
Mary did try an experimental drug that the FDA authorized for her, but her insight as an oncology nurse told her when the fight would only degrade the quality of the time she had remaining. “In most of our training as physicians, we don’t deal with this area,” Pazdur said. “This is reflected by the relatively late referrals to hospice. My wife actually told the physician, rather than the physician telling her, that it was time to go to hospice because she realized there were few options open and she wanted to move to comfort measures at that point.”
Changing With the Times
Now 67 years old, Pazdur is still a champion for reform. The OCE has embarked on multiple cutting-edge initiatives. These include reevaluating clinical trial eligibility to ensure that enrollees reflect real-world populations, promoting tumor site-agnostic indications, advancing seamless trial protocols to eliminate redundancy and inefficiency, and endeavoring to incorporate practical evidence into potential regulatory decisions. The OCE is also working to support the use of novel clinical trial designs, such as master protocols, which allow for the evaluation of multiple drugs and disease states simultaneously through a single trial or protocol.
In addition, the FDA has worked to introduce new end points, such as complete response, progression-free survival, minimal residual disease, and metastasis-free survival.
Even response as measured by circulating DNA could become a future end point if the data are supportive, Pazdur said. Along those lines, he is also supportive of incorporating patient-reported outcomes into the decision process, and the OCE has created a dedicated section to investigate this path.
“We obviously have to have the rigor around those end points,” he cautions. “Many times, patient-reported outcomes have been relegated to secondary end points, and the requisite degree of collecting data for statistical analyses of these end points has been missing. But I’m totally supportive of them being included in product labeling as primary end points of a trial when that rigor has been established.”
Many ways of measuring efficacy exist, and part of being responsive to the patient population involves recognizing what is going to be meaningful to them, such as a reduction in tumor size or a slower progression. Digging both feet into the ground and insisting on overall survival, which can take years to measure, can be a disservice to patients, he noted. “I would like my legacy to be that I was a medical oncologist who helped patients get drugs,” Pazdur said.
The individuals Pazdur has brought aboard at the FDA have evolved with the agency, and their strength and passion for oncology make going to work every day worthwhile, he said. “I’ve really developed the careers of many people who have come right out of their fellowship and right now are taking leadership positions both in the agency and in the field of oncology, and so that is my greatest accomplishment.”
References
- Oncology Center of Excellence. 20 years of OHOP NME approvals. Silver Spring, MD: Food and Drug Administration. Received November 15, 2019. Accessed December 13, 2019.
- Editorial. FDA to patients: drop dead. Wall Street Journal—Eastern Edition. September 24, 2002:240(60):A18.
- Editorial. Pazdur’s cancer rules. Wall Street Journal—Eastern Edition. July 6, 2005:246(3);A14.
- Pazdur R. An address from Dr. Richard Pazdur: How the changing landscape of oncology drug development and approval will affect advanced practice. Keynote talk presented at: Fifth Annual JADPRO Live at APSHO conference; November 3, 2017; Houston, TX.









