Publication
Article
Treatment Optimization Efforts Seek to Overcome Resistance in Advanced CML
Author(s):
Elias J. Jabbour, MD, and Matthew S. Davids, MD, MMSc, review data on asciminib and ponatinib in the post–second generation TKI setting for chronic myeloid leukemia that were presented at the 2021 ASH Meeting and Exposition.
Elias J. Jabbour, MD

Advances in the development of tyrosine kinase inhibitors (TKIs) that target the breakpoint cluster region–Abelson murine leukemia 1 (BCR-ABL1) fusion have changed the treatment landscape for patients with Philadelphia chromosome–positive chronic myeloid leukemia (CML).1 Patients of receive their initial CML diagnosis in the chronic phase, during which time disease may be controlled with treatments with approved TKIs such as imatinib mesylate (Gleevec). Second generation TKIs have elicited durable responses among patients treated in the first- and second-line setting. However, up to 20% of patients may develop resistance via acquired mutations such as the gatekeeper T315I mutation.1
Overcoming resistance mechanisms in this patient population has resulted in the development of the agents asciminib (Scemblix) and ponatinib (Iclusig). Asciminib was granted accelerated approval by the FDA in October 2021 for patients with Philadelphia chromosome–positive CML in chronic phase who were previously treat with 2 or more TKIs. The agent also received regular approval for patients with Philadelphia chromosome–positive CML in any phase who present with a T315I mutation.2 Ponatinib carries the same designations from the FDA but comes with a boxed warning for arterial occlusive events, venous thromboembolic events, heart failure, and hepatoxicity.3
In a recent OncLive The Talk™ video program, Elias J. Jabbour, MD, joined moderator Matthew S. Davids, MD, MMSc, to review data on both agents in the post–second generation TKI setting that were presented at the 63rd American Society of Hematology (2021 ASH) Meeting and Exposition. Specifically, the pair discussed recent data from the phase 3 ASCEMBL trial (NCT03106779) evaluating asciminib vs bosutinib (Bosulif) and contextualized new findings for ponatinib from a post hoc analysis of the dose-optimization phase 2 OPTIC trial (NCT02467270).4,5
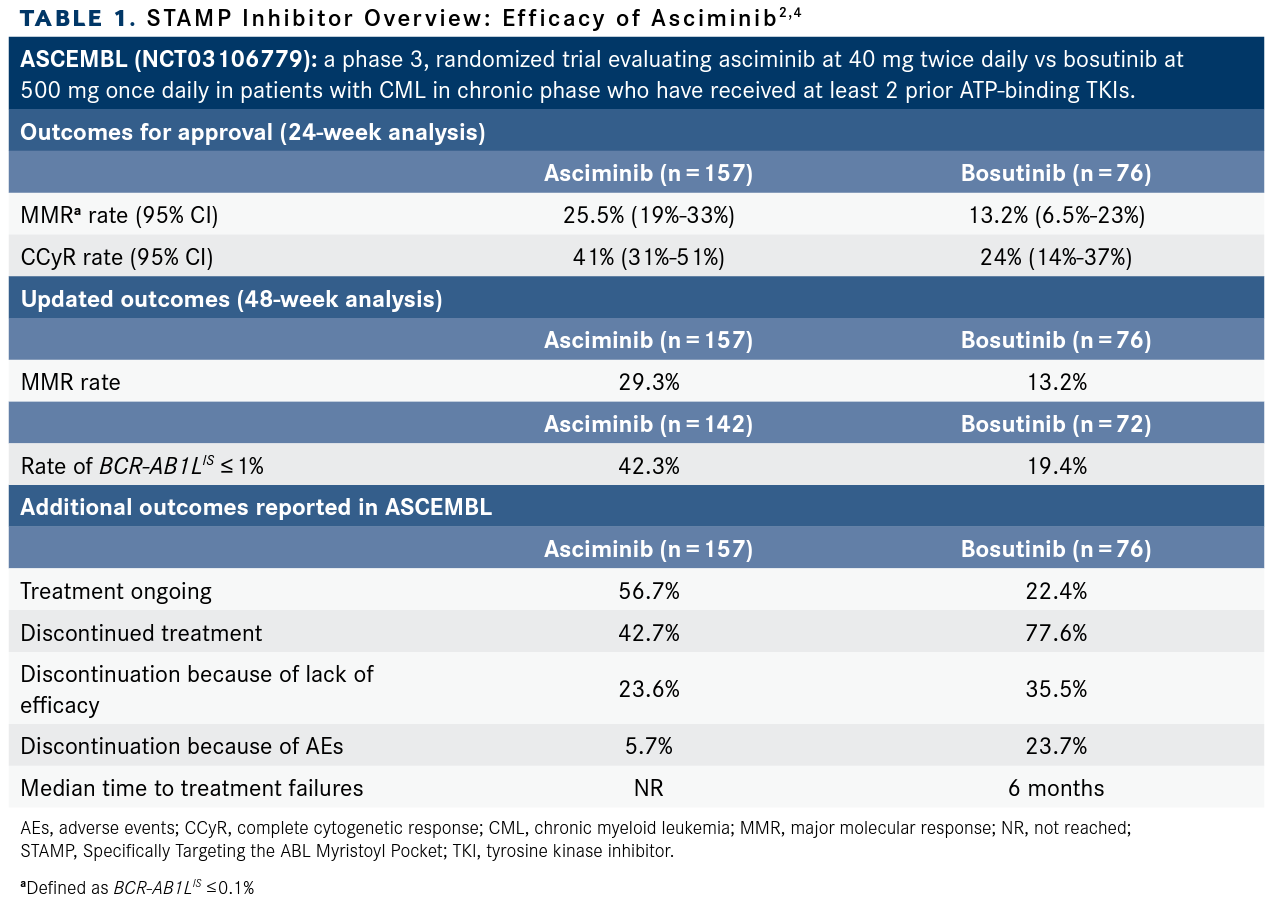
In ASCEMBL, investigators presented updated findings for patients who had at least 48 weeks of treatment with asciminib (n = 157) vs those who received bosutinib (n = 76). Major molecular response (MMR) rates at 48 weeks were 29.3% among those who received asciminib and 13.2% for those who received bosutinib (common treatment difference 16.1%; 95% CI, 5.7%-26.6%).4 Achievement of BCR-ABL1IS of 1% or less was reported among 50.5% of patients who received asciminib vs 33.7% of patients who received bosutinib. More results from the trial are highlighted in TABLE 1.2,4
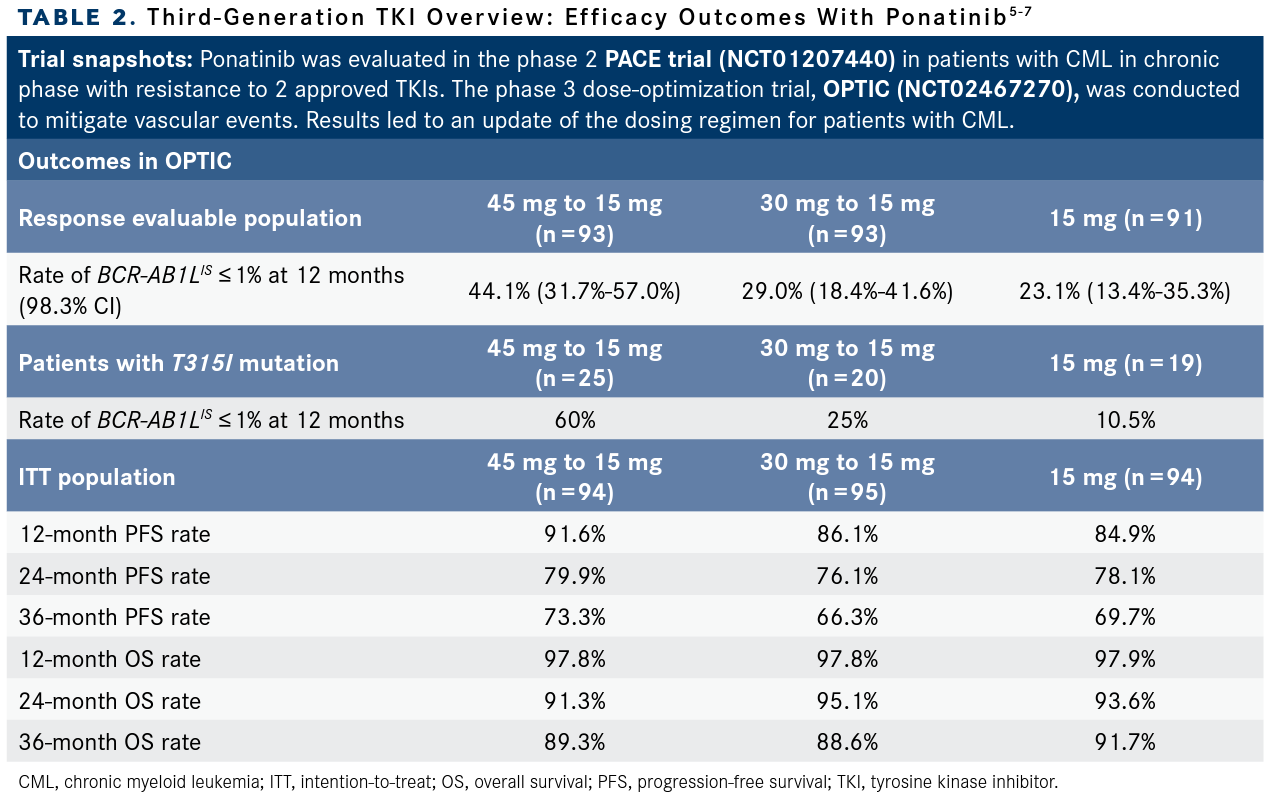
In OPTIC, updated outcomes regarding the benefit/risk analysis of different dosing strategies of ponatinib—cohort A: 45 mg to 15 mg (n = 93); cohort B: 30 mg to 15 mg (n = 93); cohort C: 15 mg (n = 91)—demonstrated that durable responses measured as BCR-ABL1IS expression 1% or less was achieved in all cohorts. Patients in cohort A experienced the highest response rate measured as BCR-ABL1IS of 1% or less at 12 months at 44.1% (98.3% CI, 31.7%-57.0%) meeting the prespecified statistical end point. Among those in cohort A who had a T315I mutation at baseline (n = 15), the response rate at 12 months was 60%. More results from the analysis are highlighted in TABLE 2.5-7
Updates from ASH 2021
Davids: We’re going to start our discussion with the efficacy and safety [data] from ASCEMBL, a phase 3 study of asciminib, which is a first-in-class STAMP inhibitor, vs bosutinib in patients with CML and chronic phase after 2 or more prior TKIs. This is an update after 48 weeks. Dr Jabbour is going take us through this. Maybe if you could start with what is a STAMP [Specifically Targeting the ABL Myristoyl Pocket] inhibitor and why is this active with a T315I mutation?
Jabbour: [To start,] I want to talk about asciminib, the new kid in block added to the treatment landscape in CML. Asciminib is different than ponatinib or other TKIs. It binds to the myristoyl pocket to inhibit BCR-ABL1, which is different than the prior TKIs, which use the ATP binding site to inhibit BCR-ABL1. It belongs to what we call the STAMP class of drugs.
Asciminib was already assessed in a phase 1 study [NCT02081378] with the recommended phase 2 dose established as 40 mg twice daily. With dose escalation, investigators determined a 200 mg twice daily dose as appropriate dose for patient harboring the T315I mutation.
ASCEMBL was a randomized phase 3 trial that randomly assigned patients with chronic phase CML who progressed following at least 2 TKIs and who did not have T315I mutations at baseline to asciminib 40 mg twice daily or bosutinib 500 mg once daily—the approved dose for patient who failed prior TKIs. The primary end point was MMR rate at 24 weeks, [data that were previously reported].
[The MMR rate was] 25.5% in favor of asciminib compared with 13.2% for bosutinib.2 This higher rate of MMR seen with asciminib led to the approval of the drug. Now, what we know as well, that patient enrolled here, they should have failed to prior TKI. If we look at the level of disease, some [individuals] had no disease whatsoever. BCR-ABL1IS of 1% or less was reported among 9.1% of patients who received asciminib compared with 5.3% of those who received bosutinib.
At 2021 ASH, we had a long-term follow-up of this study at 48 weeks and the results were still in favor of asciminib. I would like to highlight discontinuation rates with bosutinib compared with asciminib. [Results showed that] 77.6% of patients who received bosutinib had to stop the drug compared with 42.7% with asciminib. Intolerance is an issue here, but the drug was still better than bosutinib.
One good question is why [is there no study] comparing asciminib with ponatinib? That has not been done yet.
Moving on to ponatinib, the agent was approved a long time ago as a third-generation TKI effective against T315I mutations at a dose of 45 mg per day. Ponatinib was evaluated in patients who failed multiple TKIs, 2 or more, or who had a T315I mutation. So the patients who were enrolled were heavily pretreated, with 53% having received more than 3 TKIs.
The vast majority of patients were able to achieve complete response [55%] and responses were durable [not reached at 60 months follow-up]. Now with ponatinib at 45 mg per day, we did see vascular events, unfortunately. These vascular events were seen in nearly every patient at 45 mg per day and in patients at high risk of vascular events, therefore the drug was placed on hold for a short time.
Following the clinical hold, a trial called OPTIC [was designed to evaluate] dose optimization of ponatinib. When we lower the dose of ponatinib we can optimize the outcome. In OPTIC, patients who had received at least 2 TKIs were randomly assigned to ponatinib at 45 mg and reduced to 15 mg once the BCR-ABL1IS of 1% or less was obtained; 30 mg reduced to 15 mg once the BCR-ABL1IS was at 1% or less; or 15 mg [as a starting dose]. The primary end point was rate of 1% or less BCR-ABL1IS at 12 months. In this study, the investigators have shown that dose optimization from 45 mg to 15 mg was the winner. In a way, it does offer the best efficacy and the best safety. This led to the change of the label of ponatinib being administered at 45 mg with reduction to 15 mg.
Davids: That’s very helpful. I’m curious with asciminib, it’s early still, but have there been any resistance mechanisms identified yet?
Jabbour: This is very important question. In fact, in ASCEMBL we reported mutations in a binding site of asciminib. Similarly, as we have seen with the ATP binding site with the other TKIs, already patients are expressing mutations, and there was a rationale to combine asciminib with other TKIs, and those investigations are ongoing right now.
Other things to consider are that already even with the short follow-up there was a 5% rate of vascular events reported. Mechanistically, we do not know how [these are occurring], but that’s something to keep in mind. Finally, the dose of asciminib is different, whether you prescribe it for non–T315I-mutant disease, which is 40 mg twice daily, or T315I-mutant disease, which is 200 mg twice daily. Of course, price plays a role here because then you have to pay more for those with T315I mutations.
Davids: You said that probably we’re not going have head-to-head data for asciminib and ponatinib.
Jabbour: I think ideally that should have happened because we know in the third-line setting the best drug is ponatinib. We should have compared asciminib with ponatinib and the winner will get it all.
Davids: Without that comparative data, you have a patient sitting in front of you, how do you weigh the pros and cons of these 2 drugs? How do you decide?
Jabbour: Well, so far with ponatinib we have 10 years of follow-up. It’s extremely effective. I think it’s one of the most effective TKIs available. I start 45 mg in non-T315I-mutant disease and then reduce the dose and maintain the response. We also get to use this drug in Philadelphia chromosome–positive acute lymphoblastic leukemia as well, where the 45 mg reduction to 15 mg was safe and effective.
If there is a patient with vascular events who had an event recently, I would not be encouraged to use ponatinib for this patient. I may use asciminib but must be cautious. Yes, the rate of vascular events is lower, but the follow-up is still shorter. That said, I would be more inclined to use asciminib for these patients. At my institution, we have been using a using second-generation TKI up front, ponatinib, and then asciminib thereafter.
Davids: We talked a lot about both ponatinib and asciminib here. There were 2 other abstracts out of 2021 ASH that focused on ponatinib. Do you want to go into those specifically?
Jabbour: The focus of a post-hoc analysis of response to ponatinib in patients with CML by baseline BCR-ABL1 mutation [attempted to answer the question]: Do we need 45 mg for [every patient]? In the OPTIC trial, the dose was 45 mg per day for all comers and reduced to 15 mg, 30 mg to 15 mg, and 15 mg all along. Investigators did not decide based on mutation profile.
In this abstract, data were presented by mutation. Essentially the message is for patients with T315I mutations up front, the recommended starting dose is 45 mg because at the lower dose the complete cytogenetic response rates were lower [TABLE 2]. We see the advantage in progression-free survival in 45 mg per day compared with 30 mg and 15 mg. Therefore, I think for T315I-mutant disease—and I will always check for mutation at the baseline—start with 45 mg per day and reduce the dose later.
If a patient had no T315I mutation or other mutation, I still think there are advantages for 45 mg per day is my first choice, but then if an individual has no mutations and I’m concerned about hypertension or other vascular risk factors, I may use 30 mg and 15 mg, and the data are reassuring in that sense.
Finally, the last abstract presented was 2550,7 dose-modification dynamics of ponatinib in chronic phase CML from the patients in the OPTIC [and PACE] trials. In the phase 2 PACE trial [NCT01207440]—the original trial that led to the approval of ponatinib—the dose adjustment was based on adverse events: if a patient receives 45 mg and has adverse events, the dose is reduced to 30 mg and then 15 mg. We’ve shown that dose adjustment did not compromise efficacy.
In the OPTIC trial, the dose adjustment was based on efficacy, so you don’t have to wait for the safety concerns to happen to reduce the dose. By adjusting based on a response and efficacy, we had a better outcome. Therefore, the thresholding was determined in 2 ways: No. 1, you do not need full dose all along, and No. 2, reducing the dose based on dynamic of response is better than waiting for safety concerns.
This approach will preserve response and give you the best long-term outcome. Therefore, that led to the change of the label of the drug where you adjust the dose based on response. We have plenty of data on that now, and that dose reduction has become the standard of care.
Davids: Some great abstracts in CML, very nicely summarized. Another great ASH meeting for this disease as well.
References
- Sampaio MM, Santos MLC, Marques HS, et al. Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: a literature review. World J Clin Oncol. 2021;12(2):69-94. doi:10.5306/wjco.v12.i2.69
- Scemblix. Prescribing information. Novartis; 2021. Accessed March 11, 2022. bit.ly/3t3CjeX
- Iclusig. Prescribing information. ARIAD Pharmaceuticals, Inc; 2022. Accessed March 11, 2022. bit.ly/3tPVurP
- Mauro MJ, Minami Y, Rea D, et al. Efficacy and safety results from Ascembl, a multicenter, open-label, phase 3 study of asciminib, a first-in-class STAMP inhibitor, vs bosutinib in patients with chronic myeloid leukemia in chronic phase after ≥2 prior tyrosine kinase inhibitors: update after 48 weeks. Blood. 2021;138(suppl 1):310. doi:10.1182/blood-2021-152561
- Cortes J, Apperley J, Lomaia E, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138(21):20422050. doi:10.1182/blood.2021012082
- Deininger MW, Apperley J, Arthur CK, et al. Post hoc analysis of responses to ponatinib in patients with chronic-phase chronic myeloid leukemia (CP-CML) by baseline BCR-ABL1 level and baseline mutation status in the Optic trial. Blood. 138(suppl 1):307. doi:10.1182/blood-2021-145995
- Jabbour EJ, Deininger MW, Abruzzese E, et al. Dose modification dynamics of ponatinib in patients with chronic-phase chronic myeloid leukemia (CP-CML) from the PACE and Optic trials. Blood. 2021;138(suppl 1):2550. doi:10.1182/blood-2021-146175









