
The FDA has granted a fast track designation to MT-101 for use as a potential therapeutic option in patients with relapsed or refractory, CD5-positive peripheral T-cell lymphoma.

Your AI-Trained Oncology Knowledge Connection!


The FDA has granted a fast track designation to MT-101 for use as a potential therapeutic option in patients with relapsed or refractory, CD5-positive peripheral T-cell lymphoma.

The FDA has granted a fast track designation to 177Lu-edotreotide for use as a potential therapeutic option in patients with gastroenteropancreatic neuroendocrine tumors.

The novel brain-penetrant ROS1-selective inhibitor NVL-520 elicited clinical activity and was well tolerated in heavily pretreated patients with ROS1 fusion–positive non–small cell lung cancer.

The FDA has granted a fast track designation to TTI-101 for the treatment of patients with relapsed/refractory locally advanced, unresectable, or metastatic hepatocellular carcinoma.
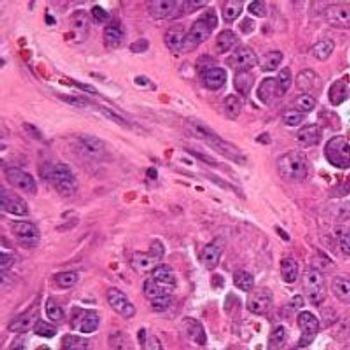
Salarius Pharmaceuticals has voluntarily paused enrollment of new patients into a phase 1/2 trial investigating seclidemstat in patients with Ewing sarcoma, sarcomas with FET-family translocations, and myxoid liposarcoma due to the death of a patient.

Ubamatamab monotherapy produced early clinical activity and an acceptable risk/benefit profile in heavily pretreated patients with ovarian cancer.

Lutetium-177-FAP-2286 produced preliminary evidence of antitumor activity with a manageable safety profile in patients with advanced or metastatic solid tumors, according to data from the phase 1/2 LuMIERE trial.

The FDA has granted an orphan drug designation to ET140203 for the treatment of patients with hepatoblastoma.

The therapeutic DNA vaccine, GX-188E, in combination with pembrolizumab produced encouraging responses in patients with human papillomavirus 16- or 18-positive, recurrent or metastatic cervical cancer who did not respond to standard-of-care treatment.

A combination regimen comprised of sotorasib and BBP-398 has been awarded a fast track designation from the FDA for use as a potential therapeutic option in adult patients with previously treated, KRAS G12C–mutated, metastatic non–small cell lung cancer.

Single-agent dostarlimab-gxly produced progression-free survival and overall survival benefits in patients with advanced or recurrent mismatch repair–deficient/microsatellite instability–high endometrial cancer.

Gilteritinib, in combination with induction and consolidation chemotherapy, generated responses in patients with newly diagnosed acute myeloid leukemia, including those with FLT3 mutations, according to data from a phase 1 trial.
DZD1516 monotherapy elicited early clinical activity and was found to be well tolerated in patients with HER2-positive metastatic breast cancer with or without brain metastases.
Ivosidenib plus venetoclax with or without azacitidine showed an expected and tolerable safety profile with durable responses and a prolonged survival rate across acute myeloid leukemia disease groups.

The FDA has granted a fast track designation to CUE-101 for use as a monotherapy and in combination with pembrolizumab in patients with human papillomavirus recurrent or metastatic head and neck squamous cell carcinoma.

The FDA has granted a fast track designation to eftilagimod alpha for use in combination with pembrolizumab as a frontline treatment for patients with stage IIIB/IV non–small cell lung cancer.

The FDA has granted a fast track designation to sapanisertib as a potential therapeutic option in patients with unresected or metastatic squamous non–small cell lung cancer whose tumors harbor an NRF2 mutation and who have previously received platinum-based chemotherapy and immune checkpoint inhibition.

MCARH109, a CAR T-cell therapy targeting the “enigmatic” GPRC5D antigen, generated remissions in 70.6% of patients with relapsed/refractory multiple myeloma.