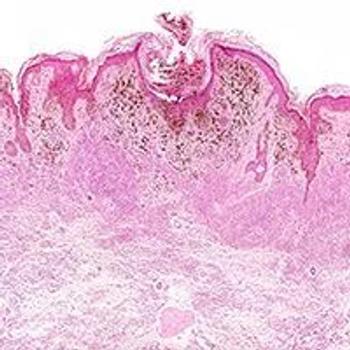
Since the FDA approval of cemiplimab in 2018, systemic therapies for the treatment of advanced or metastatic CSCC have gained prominence.

Your AI-Trained Oncology Knowledge Connection!


Since the FDA approval of cemiplimab in 2018, systemic therapies for the treatment of advanced or metastatic CSCC have gained prominence.
Anna C. Pavlick, DO, discusses the longer follow-up of the phase 2 EMPOWER-CSCC-1 study with cemiplimab in cutaneous squamous cell carcinoma.
Jennifer L. Atlas, MD, discusses the promise of cemiplimab in the treatment of patients with locally advanced cutaneous squamous cell carcinoma.
Anna C. Pavlick, DO, discusses common and uncommon adverse effects with cemiplimab in patients with cutaneous squamous cell carcinoma.

Mohammed M. Milhem, MBBS, discusses the design of an open-label, multicenter phase 1b/2 dose escalation/expansion study with AST-008 in combination with a PD-1 inhibitor, such as pembrolizumab or cemiplimab, in Merkel cell carcinoma and cutaneous squamous cell carcinoma.

Mohammed M. Milhem, MBBS, discusses the promise of the AST-008 combination in patients with Merkel cell carcinoma and CSCC who do not achieve a response with PD-1 inhibitors alone.

Nikhil Khushalani, MD, discusses cemiplimab-rwlc, which has become the preferred agent for the frontline treatment of patients with metastatic or locally advanced cutaneous squamous cell carcinoma.

Anna C. Pavlick, DO, discusses the benefit of cemiplimab for patients with cutaneous squamous cell carcinoma (CSCC), the impact of these longer follow-up data, rare, but notable, toxicities that can arise with the agent, and ongoing research efforts aimed at increasing responses to immunotherapy in the CSCC space.