
Gastrointestinal oncology experts highlight what they are most excited for at the upcoming International Society of Gastrointestinal Oncology 16th Annual Gastrointestinal Oncology Conference.

Your AI-Trained Oncology Knowledge Connection!


Gastrointestinal oncology experts highlight what they are most excited for at the upcoming International Society of Gastrointestinal Oncology 16th Annual Gastrointestinal Oncology Conference.

The FDA has approved apalutamide for the treatment of patients with metastatic castration-sensitive prostate cancer.

The FDA has granted an accelerated approval to the combination of lenvatinib and pembrolizumab for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability-high or mismatch repair deficient, and who have disease progression following prior systemic therapy but are ineligible for curative surgery or radiation.

The FDA has granted a priority review designation to a biologics license application for enfortumab vedotin for the treatment of patients with locally advanced or metastatic urothelial cancer who have received prior treatment with a PD-1/PD-L1 inhibitor and platinum-containing chemotherapy in the neoadjuvant/adjuvant, locally advanced, or metastatic setting.

A fixed-dose subcutaneous injection of pertuzumab and trastuzumab with hyaluronidase in combination with intravenous chemotherapy demonstrated noninferior pharmacokinetics compared with the standard IV infusions of the regimen in patients with HER2-positive early breast cancer, meeting the primary endpoint of the phase III FeDeriCa trial (NCT03493854).

Treatment with Toca 511 and Toca FC did not improve overall survival compared with standard therapy in patients with recurrent high-grade glioma undergoing resection, missing the primary endpoint of the phase III Toca 5 trial.

Maintenance therapy with CC-486 (oral azacitidine) led to a highly statistically significant and clinically meaningful improvement in overall survival compared with placebo in patients with newly diagnosed acute myeloid leukemia who achieved first complete response or CR with incomplete blood count recovery with induction therapy.

The investigational Trop-2–targeting antibody-drug conjugate DS-1062 demonstrated antitumor activity in unselected patients with unresectable, advanced non–small cell lung cancer.

The FDA has granted a breakthrough therapy designation to the MET inhibitor tepotinib as a treatment for certain patients with metastatic non–small cell lung cancer with MET exon14-skipping alterations.

Results from the second prespecified analysis of the TIVO-3 trial showed a hazard ratio for overall survival of 0.99 for tivozanib compared with sorafenib in patients with highly refractory metastatic renal cell carcinoma.

Treatment with nivolumab (Opdivo) was associated with a 5-year overall survival rate of 13.4% compared with 2.6% with docetaxel in patients with previously treated non–small cell lung cancer.

The question of what the accepted therapeutic strategies for de-intensification in low-risk, HPV-related oropharyngeal squamous cell carcinoma are will be debated in an upcoming discussion.

The FDA has granted a breakthrough therapy designation to capmatinib (INC280) as a first-line treatment for patients with MET exon14 skipping—mutated non–small cell lung cancer.

The combination of nivolumab plus standard temozolomide and radiation therapy did not show a statistically significant improvement in progression-free survival compared with temozolomide/radiation therapy alone in patients with newly diagnosed glioblastoma multiforme that is O6-methylguanine-DNA methyltransferase-methylated, missing one of the primary endpoints of the phase III CheckMate-548 trial.

Sipuleucel-T (Provenge) was associated with a median overall survival of 47.7 months in a subgroup of patients with asymptomatic or minimally invasive metastatic castration-resistant prostate cancer.

Risk-reducing therapies, such as tamoxifen, raloxifene, or aromatase inhibitors, should be administered to women who are at an increased risk for breast cancer and at a low risk for adverse events associated with these medications.

The European Commission has approved the combination of pembrolizumab (Keytruda) and axitinib (Inlyta) for the frontline treatment of patients with advanced renal cell carcinoma.

The FDA has granted an orphan drug designation to neratinib (Nerlynx) for the treatment of patients with breast cancer who have brain metastases.

A cluster of clinical news in biosimilars has been announced over the last few days, with pooled data sets against reference products, an expected launch of a novel study, and a cost efficiency analysis.
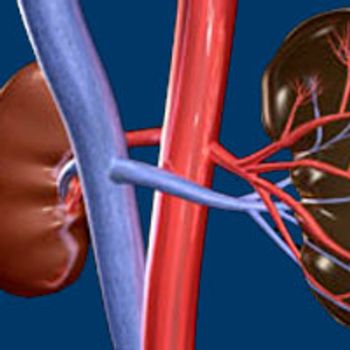
The combination of the off-the-shelf cancer immune primer ilixadencel and sunitinib elicited a 11% complete response rate in patients with metastatic renal cell carcinoma, according to topline findings from the phase II MERECA trial (NCT02432846).

Robert M. Rifkin, MD, FACP, discusses details of bringing biosimilars to market, ongoing challenges, and the need for education of these products.

Kathleen Moore, MD, combs through the various investigational strategies exploring management of resistance to PARP inhibitors in ovarian cancer.

The biosimilars realm of oncology has been surrounded by a burst of clinical and regulatory news this week, with the read out of phase III findings and extension data, legality issues, and more.

Practice-changing phase III trials including KEYNOTE-189, KEYNOTE-407, REVEL, and IMpower131, among several others, are laying the groundwork for how to treat patients with non–small cell lung cancer, specifically on an individualized basis.

ABP 798, a rituximab (Rituxan) biosimilar, demonstrated clinical equivalency to reference rituximab in patients with CD20-positive B-cell non-Hodgkin lymphoma.

The FDA has granted a priority review designation to a new drug application for zanubrutinib for the treatment of patients with mantle cell lymphoma who have received ≥1 prior therapy.

Adjuvant treatment with the trastuzumab biosimilar CT-P6 demonstrated comparable efficacy and safety to the reference product in patients with HER2-positive early breast cancer.

Selinexor in combination with twice-weekly dexamethasone led to a median overall survival of 15.6 months in patients with heavily pretreated multiple myeloma who had a minimal response or better to the novel agent compared with 1.7 months in those whose disease progressed or had unevaluable responses.

The combination of durvalumab (Imfinzi) and tremelimumab did not improve overall survival versus standard platinum-based chemotherapy in previously untreated patients with stage IV metastatic non–small cell lung cancer.

Brian Orr, MD, discusses the challenges with immunotherapy in ovarian cancer treatment while pointing to early promising data with combination regimens.