
The new indication for pertuzumab (Perjeta) as part of a three-drug neoadjuvant regimen for patients with HER2-positive metastatic breast cancer marks the first time the FDA has authorized a therapy based on pathologic complete response.

Your AI-Trained Oncology Knowledge Connection!


The new indication for pertuzumab (Perjeta) as part of a three-drug neoadjuvant regimen for patients with HER2-positive metastatic breast cancer marks the first time the FDA has authorized a therapy based on pathologic complete response.

A recognized recurring theme in many population-based cancer genetic studies is the highly provocative presence of a quite small percentage of tumors that possess specific molecular abnormalities unequivocally demonstrated to be "actionable," at least in one clinical setting.

Adjuvant therapy for patients with advanced melanoma and the optimal use of molecular testing are among the most pressing issues facing oncologists who treat patients diagnosed with the disease.

Stephen B. Baylin, MD, is conducting research in his laboratory at Johns Hopkins Medicine that aims to bring epigenetic therapy to the forefront of cancer management, in particular in gaining a better understanding of the abnormalities of chromatin and DNA methylation that may account for the development of epigenetic abnormalities during tumor development.

Prostate cancer is one of the most common forms of cancer in older men, with more than 200,000 new diagnoses per year.

After decades with few available therapies, a flurry of FDA drug approvals has provided oncologists with a variety of options for treating patients with advanced melanoma.
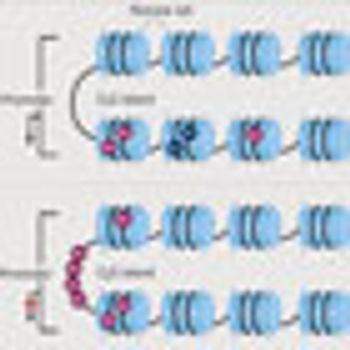
Several epigenetic therapies are already approved by the FDA, and many more are in the preclinical investigation and clinical trial phases. More than 100 agents are in various stages of development, and the field of epigenetics holds exciting implications for cancer detection, treatment, and prognosis.

Men with advanced prostate cancer who took the GnRH antagonist degarelix experienced improved disease control, fewer instances of urinary infections, and a lower risk of cardiovascular events than did those patients who took LHRH agonists.

The ability of clinicians to improve treatment outcomes that gemcitabine offers for patients with metastatic pancreatic cancer is expected to move forward now that the FDA has approved a new indication for nab-paclitaxel (Abraxane) as part of a combination regimen, according to researchers.

Marincola made a bold move earlier this year when he left a longterm position as a tenured senior investigator at the National Institutes of Health (NIH), in Bethesda, Maryland, and moved nearly 7000 miles to the Middle East to build a research program at a private hospital in Qatar.

One of the privileges of working in an academic medical center is the luxury of being able to subspecialize in the treatment of sarcoma. Nationwide, there may be 40 to 50 medical oncologists who focus on the treatment of adult soft tissue and bone sarcoma.