Oncology Business Management
Latest News
Latest Videos

CME Content
More News

There are many ideas that if followed will not just help you to "do your time" during fellowship, but contribute to the internal medicine residency program.

There is no such thing as a typical day. Our days are punctuated by the unexpected and our field is so diverse that we often change what we do over the course of our careers. Time alters us and our field.

Shrinking reimbursements and higher healthcare costs are making it difficult for community oncologists to stay in practice.

Optical biomarkers derived from nondysplastic metaplastic cells can detect the presence of high-grade dysplasia and adenocarcinoma from Barrett's esophagus.
Patients with oropharyngeal squamous cell carcinoma who had matted lymph nodes -- nodes that are connected together -- are more likely to metastasize than those without matted lymph nodes.
Treatment with tivantinib produced a 56% improvement in time-to-progression in patients with hepatocellular carcinoma.

Contrary to earlier findings, surgical breast biopsies may not be as overused as previously thought, according to a recent study published in the Journal of the American College of Radiology.

In 2011, the FDA reported 232 cancer therapies to be in limited supply. As a result, many patients have had therapies delayed or discontinued, and in some cases, patients have been left without access to appropriate alternatives.
The FDA announced plans to strengthen the supply of 2 scarce cancer drugs: methotrexate and Doxil.

Year after year, the John Theurer Cancer Center (JTCC) harnesses the newest technologies and retains world-class oncologists, nurses, and scientists to fulfill its mission of delivering extraordinary care to patients in the community.

Human errors can cause higher-than-expected medication error rates and delays in getting the proper chemotherapy doses to the patients who need them.
The Trials in Progress section supplies summaries of ongoing research in a broad range of cancer types.

It is increasingly recognized that the realm of molecular diagnostic testing in oncology is undergoing as much change, if not more, as the world of cancer therapeutics.

Radium-223, an alpha-emitting radiopharmaceutical, has been shown to improve overall survival (OS) in men with metastatic castrationresistant prostate cancer (mCRPC) in an interim analysis of the ALSYMPCA trial.
Middle-aged men taking a class of drugs called statins to reduce cholesterol levels were shown to have reduced the risk of mortality due to prostate cancer by approximately 50%, according to a new study...
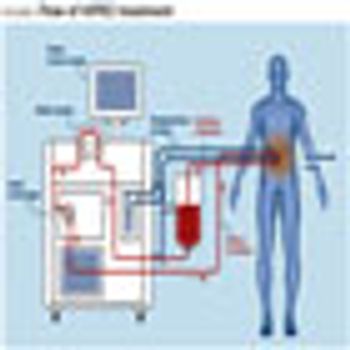
There is an ongoing debate in the oncology community about a treatment technique that involves cutting all visible cancer out of the abdomen and then flooding the cavity with heated chemotherapy drugs. The procedure -- known as cytoreductive surgery (CRS) -- followed by hyperthermic intraperitoneal chemotherapy (HIPEC), or a
Bevacizumab (Avastin) improved progression-free survival (PFS) in patients with ovarian cancer but was not shown to affect overall survival (OS), according to a new study...

This chart lists the new FDA-approved oncology drugs of 2011 and indications.

The FDA approved more new oncology drugs in 2011 than it has in a single year in more than a decade, thanks in part to priority review and streamlined evaluation procedures. While this is good news for patients, oncology and hematology specialists must tackle the challenges posed by integrating new therapies into treatment plans. Leading specialists give their perspectives on key issues.

Patients with hormone receptor-positive metastatic breast cancer (MBC) frequently respond to multiple lines of endocrine therapy but, unfortunately, resistance eventually develops.

Danish investigators are reporting that long-term cell phone use does not appear to boost the risk of central nervous system (CNS) tumors.
The investigational compound cabozantinib (formerly known as XL184) has generated much excitement in recent years for its ability to target multiple pathways involved in the development of cancer.

One of a small group of scientists who had a hand in developing Herceptin (trastuzumab) for the treatment of breast cancer, Dr. Debu Tripathy's current work is focused on understanding why some patients are resistant to the drug, and how to create more effective therapies for them.
Johnson and Johnson announced that they do not expect more Doxil (doxorubicin HCl liposome injection) to be available until sometime late in 2012...

The approval of trastuzumab, targeted directly to the human epidermal growth factor receptor (HER2)-positive, was a major advance in extending the lives of women with HER2-positive breast cancer.