Gynecologic Oncology
Latest News
Video Series

Latest Videos
Shorts



Podcasts
CME Content
More News
Alberto Farolfi, MD, PhD, discusses bevacizumab plus SOC in advanced cervical cancer and no contraindications for antiangiogenic agents.

John P. Diaz, MD, discusses the role of minimally invasive surgical approaches for the treatment of patients with uterine cancer.
The combination of metformin, letrozole, and abemaciclib was safe and active in patients with ER-positive recurrent endometrial cancer.
Neoadjuvant tislelizumab plus chemotherapy yielded responses in locally advanced cervical cancer.

Kathleen N. Moore, MD, MS, discusses how standard-of-care treatment strategies for advanced recurrent endometrial cancer have improved over time.

FDA grants type A meeting to discuss RP1 BLA in melanoma, CBP/p300 bromodomain inhibitor gets fast track status in NSCLC, and more.

Alison Schram, MD, discusses the potential clinical utility of PKMYT1-targeted agents in gynecologic malignancies.

John P. Diaz, MD, discusses the importance of uterine cancer recognition and treatment optimization in the context of Gynecologic Cancer Awareness Month.
Tisotumab vedotin is cleared in Hong Kong for the treatment of select patients with cervical cancer.
The development of ZW171 for gynecologic, thoracic, and GI cancers is being discontinued.

In case you missed any, below is a recap of every OncLive On Air episode that aired in August 2025.
The FDA has granted breakthrough therapy designation to rinatabart sesutecan for the treatment of patients with advanced or recurrent endometrial cancer who have disease progression on or following standard-of-care therapy.

Alberto Farolfi, MD, PhD, discusses real-world findings with bevacizumab plus platinum-based chemotherapy and pembrolizumab in advanced cervical cancer.

Jyoti Mayadev, MD, discusses the clinical significance of tumor area positivityas a predictive biomarker in locally advanced cervical cancer.

Linda R. Duska, MD, MPH, discusses the background of the phase 3 KEYNOTE-A18 trial of pembrolizumab plus chemoradiotherapy in cervical cancer.

Extended follow-up showed sintilimab plus anlotinib yielded durable efficacy and tolerability in PD-L1–positive recurrent/metastatic cervical cancer.

Alberto Farolfi, MD, PhD, shares results from a real-world study of first-line bevacizumab, pembrolizumab, and platinum chemotherapy in cervical cancer.

Linda Duska, MD, MPH, discusses final results of KEYNOTE A18 for pembrolizumab plus chemoradiotherapy in high risk, locally advanced cervical cancer.

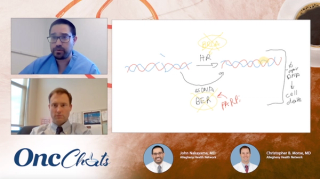
Panelists discuss how emerging antibody-drug conjugates targeting B7-H4 and folate receptors are showing unprecedented 40% to 50% response rates in recurrent endometrial cancer, representing a paradigm shift from historical treatment limitations while highlighting the exciting but challenging landscape of sequencing multiple novel therapies.

Jyoti S. Mayadev, MD, discusses a ctDNA analysis from the CALLA study of chemoradiation with or without durvalumab in locally advanced cervical cancer.
Nimotuzumab plus chemotherapy was more effective than chemotherapy alone in patients with recurrent or persistent cervical cancer.

After 3 years of follow-up, dostarlimab plus chemotherapy was effective and tolerable in patients aged 70 years or older with endometrial cancer.

Bhavana Pothuri, MD, discusses remaining unmet needs for patients with recurrent/advanced endometrial cancer.

Panelists discuss how the recent approval of avutometinib plus defactinib for KRAS-mutated low-grade serous ovarian cancer addresses a significant unmet need in this lethal disease, with the dual FAK/MEK inhibition approach showing impressive response rates despite requiring careful toxicity management.

Panelists discuss how the emerging pipeline of folate receptor-targeted antibody-drug conjugates, including rinatabart sesutecan (Rina-S) and other novel compounds, offers diverse payload options and potentially broader patient eligibility with different toxicity profiles, though sequencing strategies remain to be determined.