
The addition of relacorilant to nab-paclitaxel improved overall survival compared with nab-paclitaxel alone in patients with recurrent, platinum-resistant ovarian cancer.

Your AI-Trained Oncology Knowledge Connection!


The addition of relacorilant to nab-paclitaxel improved overall survival compared with nab-paclitaxel alone in patients with recurrent, platinum-resistant ovarian cancer.

The combination of eftilagimod alpha and pembrolizumab was found to produce an encouraging early overall survival rate of 73% at the 6-month landmark when used as second-line treatment in patients with PD-1/PD-L1–refractory, metastatic non–small cell lung cancer, according to data from part B of the phase 2 TACTI-002 trial.
Temozolomide priming followed by the combination of low-dose ipilimumab and nivolumab may produce durable clinical benefit in microsatellite stable and MGMT-silenced metastatic colorectal cancer.

The combination of abemaciclib and letrozole produced encouraging responses with an acceptable toxicity profile in patients with estrogen receptor–positive, recurrent or metastatic endometrial cancer with endometrioid histology, according to data from a phase 2 trial.
Nab-paclitaxel plus pembrolizumab produced an encouraging overall response rate with manageable safety in patients with advanced urothelial cancer who were refractory to platinum-based chemotherapy or cisplatin ineligible, according to data from the phase 2 ABLE trial.

The combination of nemvaleukin alpha and pembrolizumab led to encouraging clinical activity in patients with pretreated ovarian cancer who are resistant to platinum-based chemotherapy.
Neoadjuvant niraparib induced strong results for patients with BRCA-mutant, homologous repair deficient–positive advanced resectable ovarian cancer.

Niraparib in combination with bevacizumab was efficacious following 1 line of platinum-based chemotherapy among patients with newly diagnosed advanced ovarian cancer, regardless of biomarker status.
Oregovomab, an investigational monoclonal antibody with promising phase 2 data, is being tested in combination with paclitaxel for patients with advanced epithelial ovarian cancer in the phase 3 FLORA-5 trial.
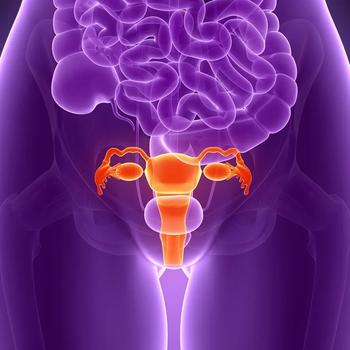
The addition of ociperlimab to tislelizumab is under investigation in the phase 2 AdvanTIG-202 trial in patients with previously treated recurrent or metastatic cervical cancer.
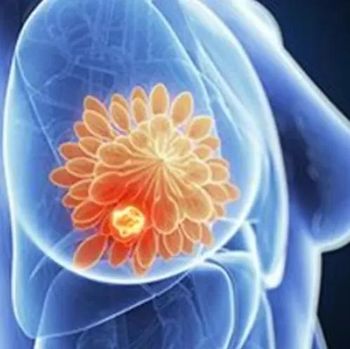
Amcenestrant did not improve progression-free survival per independent central review vs physician’s choice of endocrine treatment in select patients with locally advanced or metastatic, estrogen receptor–positive, HER2-negative breast cancer, missing the primary end point of the phase 2 AMEERA-3 trial.
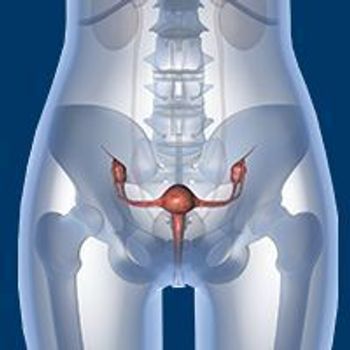
The addition of anlotinib to sintilimab appeared to be efficacious and safe when used as a second- or later-line treatment in patients with PD-L1–positive, recurrent or metastatic cervical cancer.

A subset of patients with EGFR-positive glioblastoma experienced significantly longer progression-free survival when treated with adjuvant neratinib following chemoradiation vs adjuvant temozolomide alone, although no overall PFS benefit or any overall survival improvement was seen in the overall population.

Although the addition of copanlisib to ibrutinib resulted in a high overall response rate in patients with relapsed/refractory mantle cell lymphoma, the regimen was found to have additive toxicity, according to findings from a small phase 1 trial.

The combination of ibrutinib given at 420 mg and venetoclax at 200 mg elicited an objective response rate of 93.8% in patients with relapsed/refractory mantle cell lymphoma, according to results of a phase 1 dose-finding study.

Poziotinib elicited encouraging responses when given at a daily dose of 16 mg in the first-line treatment of patients with non–small cell lung cancer with HER2 exon 20 insertion mutations.

The VEGF/Ang2–targeting bispecific nanobody BI 836880, in combination with ezabenlimab, produced antitumor activity found to be comparable to that of available third-line therapy options in patients with advanced microsatellite stable colorectal cancer.