A new generation of proteasome inhibitors, immunomodulatory agents, and deacetylase inhibitors are currently being investigated in clinical trials to treat patients with relapsed or refractory multiple myeloma.

Your AI-Trained Oncology Knowledge Connection!


A new generation of proteasome inhibitors, immunomodulatory agents, and deacetylase inhibitors are currently being investigated in clinical trials to treat patients with relapsed or refractory multiple myeloma.
A quick glance at a recent study examining the renal cell carcinoma immunotherapy AGS-003 and the FDA approval of a new noninvasive prostate health index test.
Canadian researchers are investigating standard fractionation radiotherapy with concurrent high-dose cisplatin versus accelerated fractionation radiotherapy with panitumumab in locally SCCHN.

Targeted focal therapy offers some men with localized prostate cancer a solution between active surveillance and definitive treatment with surgery and/or radiation.
Researchers have identified more than a dozen pathways and factors involving bone disease in patients with multiple myeloma who are at the highest risk of developing complications.

Cutaneous rash is a common adverse event among patients with non-small cell lung cancer who receive erlotinib and may indicate that the patient will experience a significantly greater survival benefit.

Drugs that target the programmed death-1 pathway hold considerable promise for the treatment of patients with melanoma, particularly in integrating the agents into the sequence of therapy.

Fresh strategies for grappling with the chronic, and at times critical, shortages of cancer drugs that have frustrated the oncology community for the past decade are likely to help alleviate supply problems.

Pazopanib has demonstrated activity in a majority of patients with refractory urothelial cancer, providing the first evidence that targeted therapies might have a role in the disease.
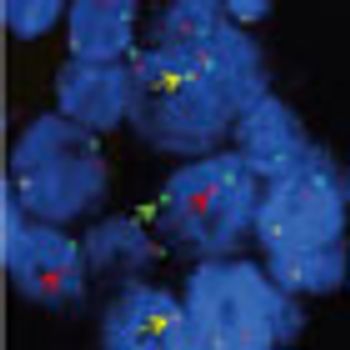
Two leading researchers discuss the role of the ALK signaling pathway and development into new ALK-targeted anticancer therapies.
There has been stunning progress as preclinical findings of the ALK gene in patients with lung cancer were rapidly translated into the availability of an FDA-approved therapeutic ALK inhibitor, crizotinib.

The ability of cabozantinib to inhibit the RET pathway contributed to the pivotal clinical trial findings demonstrating that the novel oral agent significantly improved PFS in patients with advanced MTC.

The presence of specific molecular biomarkers may assist in determining the correct morphologic diagnosis of the particular cancer being examined.