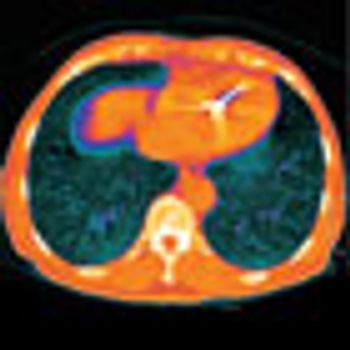
The National Lung Screening Trial (NLST), a randomized, national trial involving 53,000 current and former heavy smokers, compared the effects of 2 screening procedures for lung cancer

Your AI-Trained Oncology Knowledge Connection!


The National Lung Screening Trial (NLST), a randomized, national trial involving 53,000 current and former heavy smokers, compared the effects of 2 screening procedures for lung cancer

Useful Online Resources and Clinical Trials for Head and Neck Cancers

The search for a screening tool that can help detect lung cancers at early stages has been a focus of research aimed at the leading cause of cancer death in the United States.

There's been a flurry of activity in recent weeks when it comes to oncology drug filings at the FDA. While every application is unique, the agency is required to decide within 60 days whether a new drug application (NDA) will move forward for a full review.

The Internet has been employed in a variety of interesting ways to foster clinical or research agendas.

Useful Online Resources and Clinical Trials for Thrombocytopenia Supportive Care

Useful Online Resources and Clinical Trials for Gastrointestinal Cancers

Useful Online Resources and Clinical Trials for Childhood-Tumors

Useful Online Resources and Clinical Trials for Brain Cancer

ColoPrint, a microarray-based gene signature test, accurately identifi ed the risk of recurrence of colorectal cancer in patients with localized stage II disease, raising the potential of an exciting new prognostic tool for clinicians.

Intellisphere, the publisher of Oncology Net Guide, will launch a dynamic Internet oncology news and information hub in March, along with a rechristened version of the print magazine linked to the vibrant new content on the Website.