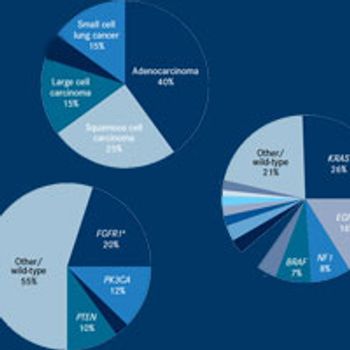
Despite these successes, driver mutations have been identified in only a minority of cases and patients with other types of lung cancer, such as squamous cell carcinoma and small-cell lung cancer, do not currently benefit from targeted therapies.

Your AI-Trained Oncology Knowledge Connection!


Despite these successes, driver mutations have been identified in only a minority of cases and patients with other types of lung cancer, such as squamous cell carcinoma and small-cell lung cancer, do not currently benefit from targeted therapies.

Ovarian cancer remains a silent and deadly tumor type with 5-year survival rates that lag far behind those of other gynecologic malignancies. Yet optimism is in the air these days as researchers focus on developing new therapies in 2 key areas: antiangiogenic agents and PARP inhibitors.

Immunotherapy continues to be one of the fastest-evolving fields in oncology. In recent years, numerous immunotherapies have shown good efficacy, safety, and durability across many tumor types, including some advanced solid tumors that have not seen meaningful treatment developments in decades.

A clinical trial that utilizes a novel radiolabeled antibody–drug conjugate to pretreat older patients with relapsed or refractory acute myeloid leukemia as part of a stem cell transplantation regimen is aimed at creating improved outcomes for a high-risk population with limited therapeutic alternatives.

Continuing research will have to evaluate whether combining serum biomarkers or other measurable clinical factors with multiparametric magnetic resonance imaging results can help make negative or inaccurate prostate biopsies a rarity rather than the norm.

Those responsible for developing and implementing governmental health policy have an extremely difficult job. Not only do they have to attempt to satisfy often highly unrealistic expectations of legislators for overall goals and timelines, but they also are frequently asked to accomplish a task with woefully inadequate funding.
Lung cancer alone accounts for 26.5% of all cancer-related mortalities in the United States, which is more than any other malignancy. Despite increased awareness that smoking is an important risk factor for lung cancer, which has reduced the number of smokers in the U.S., nearly a quarter of a million new diagnoses are expected in 2016.

The first and thus far only PARP inhibitor to gain regulatory approval, olaparib traveled a tortuous path to the 2014 FDA ruling that enabled its use for women with recurrent ovarian cancer.