
With several agents in development, small cell lung cancer, which constitutes up to 15% of lung cancers, is the focus of increased clinical investigation that may make further treatment inroads.

Your AI-Trained Oncology Knowledge Connection!


With several agents in development, small cell lung cancer, which constitutes up to 15% of lung cancers, is the focus of increased clinical investigation that may make further treatment inroads.

Women with breast cancer who have lower incomes and are members of minority populations are less likely to return to work following surgery and chemotherapy, and oncologists need to help them obtain workplace accommodations to ease their return to employment.

Sundar Jagannath, MBBS, discusses the significance the selinexor (Xpovio) approval has for patients with myeloma, the tolerability of the agent, and the management of related adverse events.

The treatment landscape changed significantly with the first melanoma clinical trials in 2001, which evaluated the CTLA-4 antibodies ipilimumab (Yervoy) and tremelimumab.

Conflicting guidelines on prostate-specific antigen testing have affected trends on disease presentation and, potentially, the treatment outcomes for many men with this disease. New agents and powerful new imaging tools have added yet more complexity to the decision process. Therefore, more investigation and cooperation on multiple levels is needed to define appropriate standards of care.
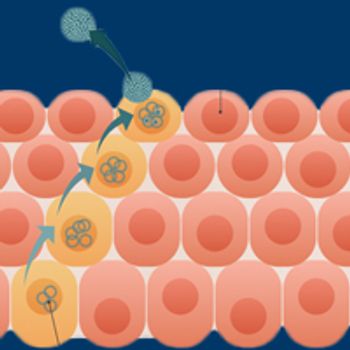
The development of therapeutic vaccines for patients with cancers associated with the human papillomavirus has emerged as a leading strategy in continuing research efforts to address the growing public health threat posed by the virus.

With the revolution in our understanding of cancer’s basic molecular biology, it is increasingly evident that subgroups of cancer originating from specific regions of the body have unique natural histories and respond to very different therapeutics. For example, the importance of BRCA mutations, which define a subset of ovarian cancers impressively sensitive to PARP inhibitors, has striking altered the management of this group of gynecologic malignancies.

Given the widespread financial impact the high cost of cancer drugs has on the healthcare system, it may be time for the FDA to take a broader approach when reviewing oncology drug applications. By also examining the cost of the drug, the FDA could shine a light on escalating drug costs and play a leadership role in creating new programs and initiatives that help patients pay for promising new therapies.

Andre Goy, MD, MS, discusses cutting-edge CAR T-cell therapy and other groundbreaking investigations, as well as his thoughts on general developments in oncology and hematology.

Although chemotherapy combinations remain standard first-line therapy for advanced or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) cancer, strategies for progressive disease have shifted to include antiangiogenic agents and immunotherapy.

The growth in the incidence and cost of treating brain metastases creates a critical challenge for physicians and cancer care centers.