
A liquid biopsy that measures tumor mutational burden showed promise as an aid for predicting benefit in patients with non-small cell lung cancer treated with a checkpoint inhibitor.

Your AI-Trained Oncology Knowledge Connection!


A liquid biopsy that measures tumor mutational burden showed promise as an aid for predicting benefit in patients with non-small cell lung cancer treated with a checkpoint inhibitor.

Weill Cornell Medicine has been awarded a five-year, $11.3 million Specialized Programs of Research Excellence grant from the National Cancer Institute to improve the detection, diagnosis and treatment of prostate cancer, a disease that affects one in six men.

The FDA has approved gemtuzumab ozogamicin (Mylotarg) for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia.

Largest cancer survivor celebration ever.

Trials evaluate combined immunotherapy with stem cell transplant to combat hematologic malignancies.

Prestigious National Cancer Institute (NCI) grant awarded for nearly $8 million to pursue unencumbered research on cancer progression.

Researchers have concluded from an analysis of multiple studies that the fecal immunochemical test has high overall diagnostic accuracy for screening asymptomatic patients at an increased risk for colorectal cancer (CRC) and moderate accuracy for diagnosing advanced neoplasia (AN).

Dr. Carlos L. Arteaga will be the new Director of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern.

Thomas Jefferson University Hospital (Sidney Kimmel Cancer Center at Jefferson) is ranked No. 20 in the Nation in the cancer specialty, according to US News and World Report.

Adam Scott Feldman, MD, MPH, urologist at Massachusetts General Hospital, assistant professor of surgery, Harvard Medical School, discusses managing patients with prostate cancer under active surveillance.

A group of researchers from several institutions in the US, including John Theurer Cancer Center, studied the development of a new, web-based intervention - Pep-Pal- to provide psychoeducation and skills for caregivers of cancer patients who are experiencing significant burden and distress including depression and anxiety.

John Theurer Cancer Center Oncology Nurses recently attended the 42nd Annual National Oncology Nursing Society’s (ONS) conference "Congress" in Denver, Colorado. Nurses in attendance presented projects they have been involved with in their area of expertise to the largest group of oncology nurses worldwide.

Watson for Oncology combined with real world data from Cota aims to help oncologists improve cancer treatment and reduce costs.

MedStar Georgetown University Hospital in Washington, D.C. in collaboration with John Theurer Cancer Center, part of Hackensack Meridian Health, Hackensack University Medical Center in Hackensack, N.J., announce the 100th blood stem cell transplant performed since the BMT program’s first patient was treated in September, 2013.

Scientists from The Wistar Institute demonstrate how a mutation in ovarian clear cell carcinoma can be exploited to design a targeted treatment.

The cost of cancer care continues to be a top concern for patients and their healthcare providers. In response, the Association of Community Cancer Centers (ACCC) has launched The Financial Advocacy Boot Camp, the first free online training program designed for all those who work to assist cancer patients in navigating financial issues related to their cancer treatment.

Alexander Kutikov, MD, has been appointed the new chief of the Division of Urologic Oncology at Fox Chase Cancer Center.

The Association of Community Cancer Centers (ACCC) and Advisory Board's Oncology Roundtable announced today their collaboration to launch a comprehensive survey that will capture the successes and challenges cancer programs across the country are facing in the current healthcare environment. Pfizer Oncology provides funding and support to ACCC for the "Trending Now in Cancer Care" survey.

The FDA has expanded the indication for the DigniCap Cooling System to be used for alopecia reduction across all solid tumors.

Joyce A. O'Shaughnessy, MD, whose research helped establish capecitabine and gemcitabine as treatments for patients with breast cancer, was honored in the Community Outreach/Education category with a 2016 Giants of Cancer Care® award, a program that OncLive® developed to recognize leaders in the field.

Expanded agreement enhances Value Pathways, providing physicians access to the most recent evidence available for five additional disease states.

With changing treatment paradigms, particularly the use of oral targeted agents, predictive value and use of prognostic factors to determine treatment choice are shifting in CLL.
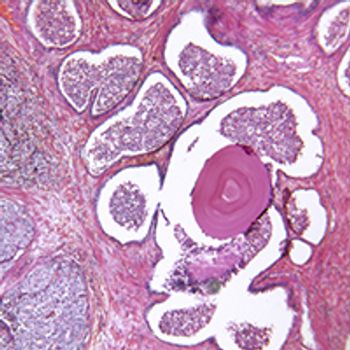
The addition of external beam radiation therapy to ADT reduced the risk of disease progression more than 70% compared with ADT alone among patients with locally advanced prostate cancer.

Inspired by the long tradition of innovation at Yale University, particularly in the life sciences, The Blavatnik Family Foundation has donated $10 million to Yale University to promote and to accelerate the development, application, and commercialization of breakthrough research in the life sciences.


The Yale School of Medicine, partnering with Yale New Haven Hospital, took the next step toward personalized medicine, cutting the ribbon on its Center for Genome Analysis on Yale’s West Campus.

Radiotherapy alone is often used to treat early-stage glottic cancer, a cancer of the vocal chords, however, the optimal radiation treatment schedule remains unknown.

In some cases, the Lassa virus starts with a fever and general weakness, moving toward headache, muscle pain, possible facial swelling, deafness, and worse. About 15 percent of patients hospitalized with severe cases die.

An early round of clinical testing shows that users of nivolumab (Opdivo), a drug sanctioned for treatment of small-cell lung cancer, more than tripled their five-year survival rate beyond the statistical average.

Findings from a clinical trial led by a researcher at NYU Langone's Perlmutter Cancer Center helped pave the way for the recent, accelerated approval by the US Food and Drug Administration of a highly effective immunotherapy as first-line treatment for patients with advanced bladder cancer who are not eligible for treatment with standard chemotherapy.