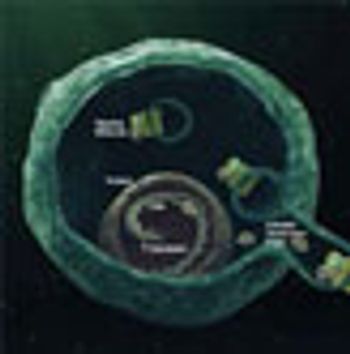
Researchers from United States and Ireland have formed a partnership to advance nanoparticle-based delivery systems that would not only drive sustained-release of cytotoxic agents, but also could improve penetration of these treatments within the tumor and allow them to continue working for days or weeks.