Oncology Business Management
Latest News
Latest Videos

CME Content
More News

D. Ross Camidge, MD, PhD, from the University of Colorado Cancer Center, explains excitement over the potential role for next-generation sequencing in cancer care.

The FDA has assigned a priority review designation to ramucirumab as a second-line treatment for patients with advanced gastric cancer.

Breast Oncology Director leads effort to honor luminaries who have helped patients survive

In many circumstances, the absence of so-called definitive evidence does not equate with the absence of evidence, just the level required by some to declare an approach to be an acceptable standard-of-care option.
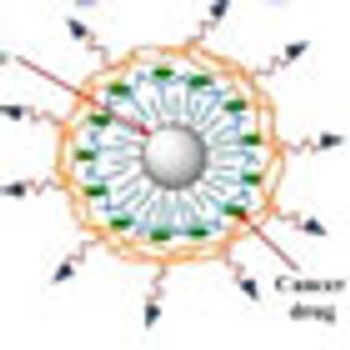
The combination of heat, chemotherapeutic drugs and an innovative delivery system based on nanotechnology may significantly improve the treatment of ovarian cancer while reducing side effects from toxic drugs.

An international research team led by scientists from Georgetown Lombardi Comprehensive Cancer Center has discovered a genetic mutation linked to low-risk bladder cancer.

Additional investment in and collaboration with ADC Therapeutics

Traditionally, payers have been increasingly more proactive in their management of drug expenditures in cancers with the highest prevalence and with the more costly therapeutics.

Findings from the first large-scale, genome-wide association study of esophageal adenocarcinoma may lead to new screening tools for those at high risk.

Governor Steve Beshear did not sugarcoat the data on May 9, 2013, when he said Kentucky would take an offer it couldn't refuse. With his state ranked worst in the nation in smoking and cancer deaths, and not far behind in heart disease, Beshear was "tired of being at the bottom."

A protein receptor for the "good cholesterol," HDL, may help make breast cancer more aggressive and offer a new target for treating the disease.

More than 95 Percent of Advanced Prostate Cancer Patients Receive Multiple Radiation Treatments for Pain Control, Despite Evidence Pointing to Equal Benefit from Single Treatments

Ten cancer programs that have developed pioneering solutions to address the challenges of treating cancer patients have received the Association of Community Cancer Centers' 2013 Innovator Awards.

The National Cancer Institute, part of the National Institutes of Health, has awarded two major grants totaling $26 million to leukemia researchers and physicians at the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis.

Offering free head and neck cancer screenings annually to the community not only has the possibility of early detection, but also the opportunity – particularly in an urban city – to increase a person's understanding of risk factors that cause cancer, according to a new study from Henry Ford Hospital in Detroit.

In response to the growing trend of hospital consolidation, including questions about cancer care decision-making, cost impacts, and delivery of care, the ACCC Institute for the Future of Oncology has released a new white paper that provides perspectives on how these alignments are affecting cancer care providers, cancer programs, and the patients they serve.

David P. Carbone, MD, PhD, a professor in the Division of Medical Oncology at the Ohio State University Comprehensive Cancer Center, discusses potential biomarkers in lung cancer.

Merrimack Pharmaceuticals, Inc. announced today that the last patient has been enrolled in the second cohort of a two-cohort randomized Phase 2 clinical trial of MM-121 in combination with paclitaxel in the neoadjuvant setting of HER2-negative breast cancer.

Increasing financial and administrative pressures-exacerbated by the federal sequester and continued rollout of the Affordable Care Act-have created instability and uncertainty for oncology practices nationwide.

Clifford A. Hudis, MD, ASCO president, chief, Breast Cancer Medicine Service, attending physician, Memorial Sloan-Kettering Cancer Center, comments on exploratory biomarker observations from the BOLERO-3 trial.

Initiative targets net reduction in annual operating expenses of approximately $2.5 billion by the end of 2015, this includes a plan for new workforce reductions of approximately 8,500 positions, in addition to pending, previously announced reductions.

Pertuzumab in combination with trastuzumab and chemotherapy has become the first FDA-approved neoadjuvant treatment for patients with breast cancer