Genitourinary Cancers
Latest News
Latest Videos

CME Content
More News

Dr. Julie Brahmer, from the Sidney Kimmel Comprehensive Cancer Center, on the PD-1 Immunotherapy BMS-93655 Clinical Trial

Dr. Suzanne Topalian, from Johns Hopkins University School of Medicine, on the PD-1 Targeted Therapy BMS-936558

Study finds that more than two-thirds of patients with metastatic kidney cancer prefer pazopanib over another FDA-approved treatment, sunitinib.

In men with primary squamous cell carcinoma of the penis who have undergone surgery that removed all glanular epithelium a maximum 12-month follow-up period is recommended.

When you consider how complex the system is, and how little training physicians get in medical school about coding, it stands to reason that practice leadership must emphasize its importance.

It is with great pleasure that I welcome you to the inaugural issue of Urologists in Cancer Care.

An interview with Brian Rini, MD, whose research has focused on RCC, prostate, and other genitourinary cancers, as well as on antiangiogenic therapy and immunotherapy.

The concept of dose titration in patients with mRCC who fail to achieve therapeutic levels on axitinib was validated by a secondary analysis.

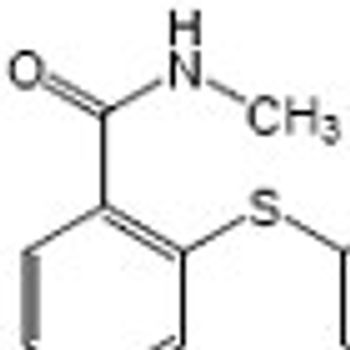
The FDA approved axitinib for the treatment of patients with advanced RCC who have failed to respond to another drug.

Axitinib has shown promising results in phase II and III trials, including the AXIS trial in patients with metastatic clear cell RCC who failed on other treatments.

The investigational drug, tivozanib, showed improved rates of PFS in patients with advanced RCC than the already-approved sorafenib.
ODAC unanimously voted to recommend the approval of axitinib for the second-line treatment of patients with advanced renal cell carcinoma.
The level of C-reactive protein (CRP) expressed in the tumor specimens of patients with renal cell carcinoma (RCC) may serve as a widely available biomarker for postoperative prognosis of localized disease.

Dr. Brian I. Rini from the Cleveland Clinic Discusses the VEGF Targeted Agent Axitinib

Investigators have documented gross under-use of established recommendations for the management of high-grade noninvasive bladder cancer.

The use of nonaspirin nonsteroidal antiinflammatory drugs (NSAIDs) is associated with an increased risk of renal cell carcinoma (RCC).

An interview with Neal D. Shore, MD, discussing expanding the scope of cystoscopy and the optical imaging agent Cysview.

Dr. Brian I. Rini from the Taussig Cancer Institute Describes Hypertension as a Biomarker in RCC
Dr. Brian Rini from Taussig Cancer Institute on the Progression of Advanced RCC Therapy

Dr. Brian I. Rini from the Cleveland Clinic on Standards of Care for Advanced RCC

Study results reveal that only a small fraction of patients with high-grade, noninvasive bladder cancer receive the appropriate treatment.

Dr. Brian I. Rini from the Taussig Cancer Institute Explains the Axitinib Integration Process
Dr. Brian Rini from Taussig Cancer Institute explains the Axitinib and Sorafenib AXIS Trial VEGF Potency Focus

Dr. Brian Rini from the Cleveland Clinic Taussig Cancer Institute on the Axitinib and Sorafenib Trial Hazard Ratio

Dr. Brian Rini from the Cleveland Clinic Taussig Cancer Institute on the Results From His Axitinib and Sorafenib Trial