
Although it’s easy to become jaded about the prospects for positive change in any bureaucracy, we are feeling decidedly upbeat about the developments underway on the federal level concerning the cancer research behemoth.

Your AI-Trained Oncology Knowledge Connection!


Although it’s easy to become jaded about the prospects for positive change in any bureaucracy, we are feeling decidedly upbeat about the developments underway on the federal level concerning the cancer research behemoth.

In recent years, advancements in the treatment of patients with metastatic melanoma have proceeded in 2 areas: targeted therapies and immunotherapies.

In August 2016, the FDA approved pembrolizumab for patients with platinum-refractory squamous cell carcinoma of the head and neck. Not only was it the first immunotherapy approved for head and neck cancer (HNC), but it marked the first new drug approval for HNC in the United States in 20 years.

Two of the most noteworthy developments in the oncology field during 2016 were the continued expansion of checkpoint blockade immunotherapy agents into more cancer types and the federal government’s plans for funding and remaking the research paradigm.

Don M. Benson Jr, MD, PhD, discusses the clinical development of KIR-targeted therapies.
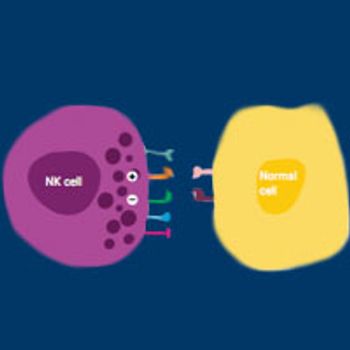
Although T cells have commanded most of the attention in the burgeoning immuno-oncology field, there is a growing appreciation that other immune cells have important roles in tumor surveillance and would represent attractive therapeutic targets.

An immunotherapy combination that adds the interleukin-10 agonist AM0010 to FOLFOX chemotherapy will be evaluated in a phase III trial for patients with metastatic pancreatic cancer that could introduce a new modality to the treatment paradigm for the malignancy.

Negative reports evaluating molecularly selected agents should not derail the process of developing future therapies by employing a precision medicine approach.