Immunotherapy and You: Understanding the PD-L1 Blockade
In this fifth episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, discuss PD-1 blockade, potential biomarkers of response to treatment with immunotherapy, and the potential for these agents in the neoadjuvant setting.
Episodes in this series

In this fifth episode of OncChats: Immunotherapy and You, John Nakayama, MD, of the Division of Gynecologic Oncology, Allegheny Health Network, and assistant professor of OBGYN at Drexel University, and Ali Amjad, MD, medical hematologic oncologist, Allegheny Health Network, discuss PD-1 blockade, potential biomarkers of response to treatment with immunotherapy, and the potential for these agents in the neoadjuvant setting.
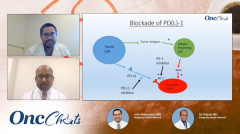
Nakayama: We've talked a bit about checkpoint inhibition at this point, but I don't think we've really gone over exactly how it works. I put together this little slide, and I would love your take on it, as well. I feel like PD-1, PD-L1, is kind of the prototypical immune checkpoint, and the way that I look at it, is you're turning that amp up to 11. You're just really turning off the 'off.' As such, you have this double-negative situation and you're ramping up the immune system. Do you have a different way that you think about it?
Amjad: Well, my patients ask me all the time, 'How does this work, and how is it different from chemotherapy?' We can talk about biomarkers and how they are the targets and how the receptors work, but the simplistic way to think about this is: We have a cancer cell on 1 side and we have the T cell on the other and they're supposed to recognize and interact but then, there's a curtain in between. That curtain is actually created by the cancer cell; that curtain, we call PD-1 or PD-L1. The T cell is just behind that curtain, but it doesn't know the cancer is right there as well. So these blockers actually lift that curtain away, so that the T cell can interact with the foreign cancer cell and eradicate it. These are checkpoints, as we discussed before; these are 'stop' symbols that are created [as] a part of the immune response. We just have to sometimes release those 'stop' signals so that the T cells don't have a false sense of security that nothing is wrong, when the cancer is right there in front of them.
Nakayama: Yeah, I couldn't agree more. You mentioned biomarkers a couple of times, and how that's so important because patients who don't have the right biomarkers are much less likely to respond to these agents. Could you talk to me a little bit about what some of those biomarkers are and how they influence who gets treatment with immunotherapy and who doesn't?
Amjad: Yeah, so these biomarkers are not perfect either. We know that immunotherapy will work when the T cell will recognize something that is not a self antigen. Whenever that creates neoantigens or new molecules that are considered foreign, would in theory, be addressed by the immune system. There are things like mismatch repair deficiency [and] tumor mutational burden [TMB] that tend to create neoantigens. We'll talk more about that; it becomes pertinent in [tumors] like endometrial [cancer or] colon cancer. Certainly, TMB is also a good biomarker. [It makes sense that] PD-1 or PD-L1 expression would be a biomarker for this, although in many cases, we do see that patients who have tumors that are PD-L1 negative [can] also respond to immunotherapy in some cases.
Nakayama: Yeah, and it's not as easy as you would think. It's not [a situation where you just check if they] have PD-1 or PD-L1 or whatnot when [you] look at [the] tumor. They actually will sometimes make these combined positive scores [CPS], and there are other scoring systems that will assign a percentage. Have you seen that in your practice, as well?
Amjad: Yeah, we have. There's a lot of ways in which we can assess this biomarker. Again, the thing to recognize there is that this biomarker is also dynamic. We use the tissue specimen from a few months, few weeks, sometimes a few years ago, to make a decision on what's happening right now with the cancer itself. [That being said,] PD-L1 can be inducible; it can go away. [As such,] there's another whole layer of complexity that comes with it. However, things like CPS [and] tumor proportion score, we [use these to] try to see this biomarker in the cancer cell and the surrounding cells and come up with a percentage or an estimate of how effective the blockade will be with these agents.
Nakayama: It seems like, at least in most of [the] cancers [that I see], they [respond to these agents if they have a PD-L1 expression of] at least a 1%. However, [a] higher percentage seems to be correlated with a higher response rate. Is that what we see with other tumors, as well?
Amjad: We do. For the most part, 1% or higher is considered a positive score. You know, in lung cancer, for example, 50% or higher would mean that they can try to skip chemotherapy altogether and use immunotherapy alone. However, we haven't quite seen that in other cancers yet.
Nakayama: That's actually a really great point, and were talking about this the other day. At this point, we've been talking about how [immunotherapy is] used in the adjuvant setting, so after [a patient has undergone] surgery for [their] cancer or after something else [has been done]. Do you see these [agents] moving forward in the lines? Do you see this moving toward maybe before [a patient gets] surgery or replacing chemotherapy altogether?
Amjad: Certainly. We have had some approvals this year earlier. Nivolumab [Opdivo], for example, is approved in the neoadjuvant setting for lung cancer. As such, there will be situations where you can try to maximize response before a complex surgery so that the patients can [get to] surgery more easily. [Immunotherapy] has replaced chemotherapy in some situations, as well. We will have to see if we can, in the future, somehow also replace surgery altogether, with something like immunotherapy.
Check back next Wednesday to view the next segment in this series.