
After decades of use, a routine test used for diagnosing prostate cancer has come under fire after a government panel questioned its safety.

Your AI-Trained Oncology Knowledge Connection!


After decades of use, a routine test used for diagnosing prostate cancer has come under fire after a government panel questioned its safety.

The Z11 study suggests that there is no difference in survival among patients with breast cancer who did not undergo a recommended surgical treatment.

When it comes to market capital, Pfizer, Inc. tops a list of major companies in the biosciences market.

If IKK kinase, a group of inhibitory proteins, activity is not regulated properly, tumors in the body have the ability to migrate and proliferate.
The FDA commissioner announced the withdrawal of its approval for bevacizumab (Avastin) to treat patients with breast cancer.
The FDA approved ruxolitinib (Jakafi) as the first drug to specifically treat patients with myelofibrosis, a bone marrow disease.
Cetuximab (Erbitux) was approved by the FDA for treating patients with late-stage head and neck cancer in combination with chemotherapy.

The following cases were discussed at the 10th International Congress on the Future of Breast Cancer, which was held in Coronado, California, from August 4-7, 2011.

ASCO endorses a Canadian agency's outlook on ovarian ablation, chemotherapy limits for stage IV lung cancer patients, and supportive care antiemetic therapies.

Johnson & Johnson is warning doctors that the demand for the chemotherapy drug Doxil will likely not be met for months to come.
A late phase metastatic colorectal cancer trial was stopped after demonstrating a statistically significant improvement in OS.

As millions of Americans grapple with changes in Medicare and other health insurance coverage, thousands of oncologists could find their medical practices threatened by insolvency.

Two trials are underway that not only address bone-related issues but might improve survival rates for patients.

Vitamin E may increase the risk of developing prostate cancer in men who have taken the supplement for 5 years or longer.
HER2-positive women can be effectively treated with Herceptin (trastuzumab injection) without the use of Adriamycin.

Dendreon estimates that only about 25% of eligible prescribing physicians were aware that Medicare had decided to cover Provenge for its on-label use of treating prostate cancer.

BIND, based in Cambridge, Massachusetts, was founded in 2007 by two researchers with experience in the field of targeted nanoparticles.

Through the success of Revlimid and other drugs, Celgene has become a respected and powerful player in the biopharmaceutical industry in recent years.

Brentuximab vedotin showed very positive results in lymphoma patients in several early trials, prompting the FDA's Oncologic Drugs Advisory Committee to unanimously recommend accelerated approval for the drug.
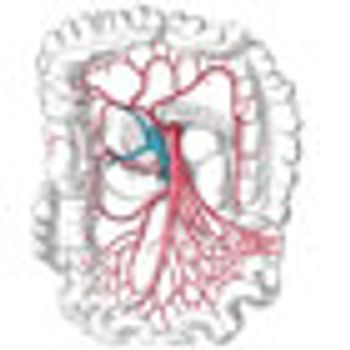
A greater number of lymph nodes are being screened in colon cancer but this has not led to more diagnoses of node-positive colon cancer.

While smoking and lung cancer have always been closely linked, recent advances in research have begun to unravel the genetic code behind the disease.
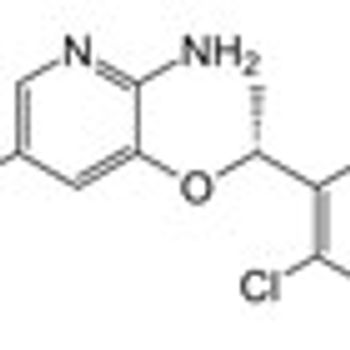
On Friday, August 26, 2011, the FDA approved crizotinib (Xalkori) to treat ALK-positive patients with late-stage non–small cell lung cancer (NSCLC).

The FDA announced that brentuximab vedotin has been approved for the treatment of Hodgkin lymphoma and systemic anaplastic large cell lymphoma.
On July, 29, 2011, a US appeals court sided with Myriad Genetics, Inc's patents on the BRCA1 and BRCA2 genes.
The tumor suppressor gene PTEN has been linked to the removal of DNA damage from UVB radiation, a major risk factor in the development of skin cancer.
Three new genetic mutations have been identified in patients with Barrett's esophagus (BE) and esophageal adenocarcinoma (EAC).