
Jae Park, MD, discusses the FDA approval of obe-cel for adult patients with relapsed or refractory acute lymphoblastic leukemia.

Your AI-Trained Oncology Knowledge Connection!


Jae Park, MD, discusses the FDA approval of obe-cel for adult patients with relapsed or refractory acute lymphoblastic leukemia.
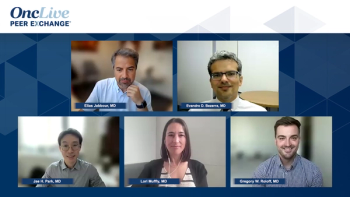
The key opinion leaders provide concluding insights and essential recommendations regarding the management of relapsed/refractory acute lymphoblastic leukemia.

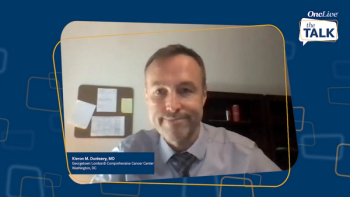
Key opinion leaders address the availability of brexucabtagene autoleucel (brexu-cel) at their institutions and outline procedures for ensuring risk evaluation and mitigation strategy compliance and comprehensive training across multidisciplinary teams.

Acute lymphoblastic leukemia specialists explore resources and strategies to address the logistical hurdles of CAR-T therapy implementation, while also examining patient support programs designed to enhance treatment accessibility and adherence.

Experts in acute lymphoblastic leukemia examine the logistical challenges associated with CAR T-cell therapy delivery and assess the frequency of patient attrition due to manufacturing delays.

The panel explores laboratory parameters routinely monitored for CRS and ICANS, including monitoring frequency, and discusses prophylactic measures and safety management strategies employed to address these complications in CAR-T therapy.

Experts evaluate the frequency of severe cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) in CAR-T therapy, comparing their incidence to that observed with other immunotherapies such as bispecific antibodies.

The panel examines their strategies for consolidation or maintenance treatment following CAR T-cell therapy, while also considering whether CAR T-cell therapy can function as a standalone treatment modality.

Medical experts specializing in acute lymphoblastic leukemia explore emerging trends and potential future developments for CAR T-cell therapy in treating relapsed or refractory B-cell ALL.

The panel explores the potential impact of obecabtagene autoleucel on the treatment landscape and decision-making process for acute lymphoblastic leukemia, contingent upon its FDA approval.

A medical expert reviews the FELIX study, highlighting its key findings and characteristics while comparing and contrasting it with the ZUMA-3 trial in the context of CAR T-cell therapy for acute lymphoblastic leukemia.

The acute lymphoblastic leukemia experts analyze the sequencing of CAR T-cell therapy for patients with and without prior blinatumomab exposure, addressing potential concerns associated with using blinatumomab before CAR T-cell treatment.

Medical experts examine the importance of bridging therapies preceding CAR T-cell infusion, addressing their frequency of use and preferred therapeutic agents for this purpose.

The panel evaluates key patient characteristics and clinical factors influencing the decision to recommend CAR T-cell therapy as a treatment option in relapsed or refractory acute lymphoblastic leukemia.

A medical expert elaborates on the therapeutic role of brexucabtagene autoleucel in the management of relapsed or refractory B-cell acute lymphoblastic leukemia.

The panel examines the current positioning of CAR T-cell therapy within the treatment paradigm for Relapsed / Refractory Acute Lymphoblastic Leukemia, considering the distinctions between Philadelphia chromosome-positive and negative patients.

The panelists explore therapeutic strategies for adult patients diagnosed with relapsed or refractory B-cell acute lymphoblastic leukemia.

Medical experts outline the typical timeline for CAR T-cell therapy, encompassing the stages from initial cell collection through manufacturing to final patient infusion.

Key opinion leaders succinctly compare the dosing and lymphodepletion protocols for Brexucabtagene autoleucel, Tisagenlecleucel, and Obecabtagene autoleucel.

Dr. Lori Muffly explains the general mechanism of action in CAR T-cell therapy and compares the distinct features of Brexucabtagene autoleucel (Brexu-cel), Tisagenlecleucel (Tisa-cel), and Obecabtagene autoleucel (Obe-cel).

Evandro D. Bezerra, MD, provides an overview of current CAR T-cell therapies for relapsed/refractory B-cell acute lymphoblastic leukemia and explores emerging CAR T products under investigation.
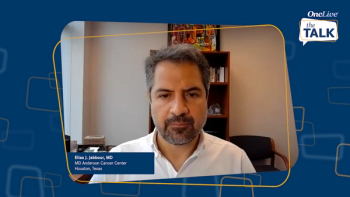
Shared insight from expert oncologists on key clinical trial data in lymphomas from the ASCO 2022 and EHA 2022 Annual Meetings.
Expert oncologist perspectives on key clinical trial data in acute lymphocytic leukemia from the ASCO 2022 and EHA 2022 Annual Meetings.

Jae H. Park, MD, discusses emerging CAR T-cell therapies in hematologic malignancies.

Jae H.Park, MD, discusses potential alternate CAR T-cell targets in B-cell malignancies.

Jae H. Park, MD, discusses encouraging data with CAR T-cell therapy in acute lymphoblastic leukemia.
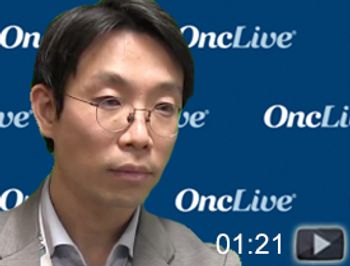
Jae H. Park, MD, attending physician, Leukemia Service and Cellular Therapeutics Center, Memorial Sloan Kettering Cancer Center, discusses the use of CAR T cells in the treatment of patients with acute lymphoblastic leukemia.
Jae H. Park, MD, discusses a particular challenge he sees in using CAR T-cell therapies in patients with acute myeloid leukemia.
Published: July 11th 2024 | Updated:

Published: August 8th 2024 | Updated:
Published: July 13th 2022 | Updated:

Published: July 18th 2024 | Updated:

Published: July 25th 2024 | Updated:

Published: July 11th 2024 | Updated: