
Some patients with mantle cell lymphoma can delay treatment without experiencing negative outcomes, according to findings from a population-based study.

Your AI-Trained Oncology Knowledge Connection!


Senior Editor, OncLive®
Jason Harris has worked in journalism for more than 20 years, including stints at daily newspapers and niche publications for oncology and cardiology. He is a senior editor for OncologyLive® and managing editor for Oncology Fellows and the annual Giants of Cancer Care® album. He also contributes to the OncLive On Air and OncFellows podcasts. Email: jharris@onclive.com

Some patients with mantle cell lymphoma can delay treatment without experiencing negative outcomes, according to findings from a population-based study.

The European Commission has approved midostaurin (Rydapt) for adults with newly diagnosed FLT3-positive acute myeloid leukemia and advanced systemic mastocytosis.

After a median of 10 years’ follow-up, investigators with the ICECaP Working Group have concluded that there is a strong correlation between metastasis-free survival and overall survival in patients with localized prostate cancer.

Patients who received maintenance therapy with rituximab (Rituxan) following autologous stem cell transplantation as treatment for mantle cell lymphoma had a survival advantage, according to results from a retrospective single-center study.

In a pooled analysis, investigators determined that ibrutinib (Imbruvica) induced an objective response rate of 66% in patients with mantle cell lymphoma.

The FDA has approved a cabazitaxel (Jevtana) regimen of 20 mg/m2 every 3 weeks in combination with prednisone for the treatment of men with metastatic castration-resistant prostate cancer.

BLU-554 induced an overall response rate of 16% in patients with FGF19-positive hepatocellular carcinoma.

A 15 mg/kg dose of rabbit anti-T-lymphocyte globulin was associated with similar graft-vs-host-disease, less nonrelapse mortality, and less disease recurrence compared with a 30 mg/kg dose for children with hematological malignancies.

The FDA approved the first biosimilar for the treatment of cancer. ABP-215 (bevacizumab-awwb; Mvasi), a biosimilar for bevacizumab (Avastin), is indicated for the treatment of colorectal, lung, brain, kidney, and cervical cancers in adult patients.

High-intensity local radiation combined with systemic therapy improved overall survival compared with systemic therapy alone in patients with head and neck squamous cell carcinoma.

Administering high-dose cytarabine to children with myeloid leukemia of Down syndrome during the second chemotherapy induction cycle resulted in improved event-free survival and overall survival compared to results observed in previous research studies.

The FDA has accepted a biologics license application for the rituximab biosimilar Rixathon, according to Sandoz, the company developing the treatment.

The FDA has granted a breakthrough therapy designation to cemiplimab (REGN2810) for the treatment of adults with metastatic cutaneous squamous cell carcinoma (CSCC) and adults with locally advanced and unresectable CSCC.

The FDA has placed clinical holds on multiple trials in Celgene’s FUSION program, which is exploring regimens combining the PD-L1 inhibitor durvalumab with immunomodulatory and chemotherapy agents across several hematologic malignancies.

18Fluorodeoxyglucose-positron emission tomography/computed tomography scanning reliably detected residual neck disease in newly diagnosed patients with locoregionally advanced head-and-neck squamous cell carcinoma.

The FDA has placed partial clinical holds on 3 trials assessing nivolumab (Opdivo)-based combinations in patients with relapsed/refractory multiple myeloma.

The phase Ib/II Study 111 examining the combination of pembrolizumab and lenvatinib in women with metastatic endometrial carcinoma has been expanded based on positive interim results.

Results from an analysis of 3D tumor volume measurements shows papillary thyroid cancers ≤1.5 cm grew slowly during a period of active surveillance, suggesting that surgery may not be necessary for all patients.

Icotinib (Conmana) was associated with a 3.3-month increase in median progression-free survival compared with chemotherapy in patients with stage IIIB/IV non–small cell lung cancer.

The FDA placed clinical holds on 2 phase I trials investigating a gene-edited allogeneic CAR T-cell (UCART) therapy known as UCART123.

Z-endoxifen, a potent derivative of the drug tamoxifen, induced tumor shrinkage in women with ER-positive metastatic breast cancer who had progressed on standard anti-estrogen therapies.
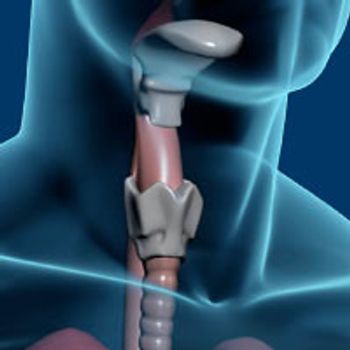
Cabozantinib (Cabometyx) demonstrated durable activity in patients with radioiodine-refractory differentiated thyroid cancer who progressed on VEGFR-targeted therapy.

The FDA has granted LN-144, an adoptive cell therapy that uses tumor-infiltrating lymphocyte technology developed by Iovance Biotherapeutics, a fast track designation for the treatment of patients with advanced melanoma.

Pembrolizumab induced an overall response rate of 33% in patients with extensive-stage small cell lung cancer.

The FDA has granted a priority review to a supplemental new drug application for bosutinib for the first-line treatment of patients with Philadelphia chromosome-positive chronic myeloid leukemia.

The investigational HER2-targeting antibody-drug conjugate DS-8201 has received an FDA breakthrough therapy designation for the treatment of patients with HER2-positive, locally advanced, or metastatic breast cancer.
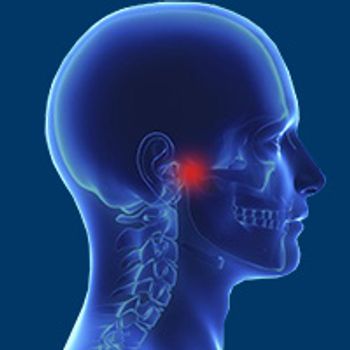
Pembrolizumab (Keytruda) induced an overall response rate of 25.9% and was well tolerated in patients with PD-L1–positive recurrent or metastatic nasopharyngeal carcinoma.

The FDA has granted a priority review to a supplemental biologics license application for obinutuzumab (Gazyva) in combination with chemotherapy, followed by obinutuzumab alone, for the first-line treatment of patients with follicular lymphoma.

The European Commission has approved tivozanib for the treatment of patients with advanced renal cell carcinoma.

Results from the PROSELICA trial showed that 20 mg/m2 of cabazitaxel (Jevtana) was as safe and effective as the 25 mg/m2 dose for postdocetaxel patients with metastatic castration-resistant prostate cancer.