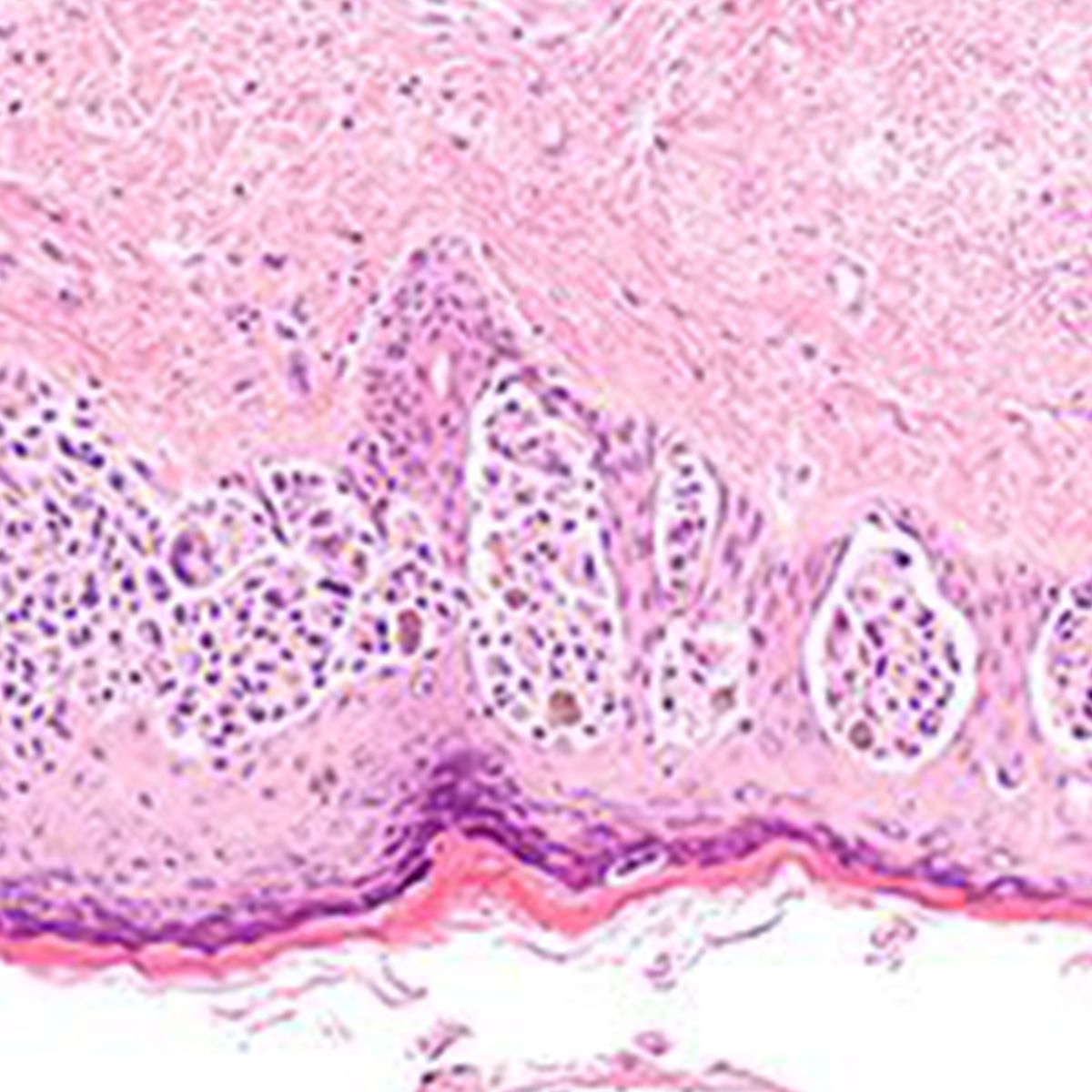
Melanoma & Skin Cancer
Latest News
Latest Videos

CME Content
More News

Ahmad Tarhini, MD, PhD, discusses the hypothesis for investigating high-dose bolus IL-2 plus an anti–CTLA-4 agent in patients with advanced melanoma.

Ahmad Tarhini, MD, PhD, discusses the use of high-dose bolus IL-2 with ipilimumab followed by nivolumab in advanced melanoma following prior progression.
Treatment with VP-315 led to reductions in tumor size in patients with basal cell carcinoma.

Georgina Long, MBBS, PhD, FRACP, discusses unmet needs for BRAF-mutant melanoma, highlighting key insights from the phase 3 NADINA trial.
A sNDA seeking the approval of toripalimab for frontline use in patients with unresectable or metastatic melanoma is under review by the NMPA.

A tumor-informed ctDNA assay showed high sensitivity and specificity as well as potential for ctDNA to be used as a prognostic biomarker during surveillance in Merkel cell carcinoma.

Integrating 15-GEP with PRAME expression status into a 4-group prognostic classification system showed prognostic accuracy in uveal melanoma.

The addition of BNT111 to cemiplimab has produced clinical activity in anti-PD-(L)1 relapsed/refractory unresectable stage III or IV melanoma.

The FDA has accepted the resubmission of a BLA for cosibelimab in advanced or metastatic cutaneous squamous cell carcinoma.

Adjuvant nivolumab plus ipilimumab significantly improved 3-year DMFS rates compared with historical controls in patients with high-risk uveal melanoma.

Neil D. Gross, MD, FACS, discusses the neoadjuvant use of cemiplimab for the treatment of cutaneous squamous cell carcinoma.

The FDA has granted fast track designation to OBX-115 for advanced melanoma that is refractory to or relapsed after immune checkpoint inhibitors.

Nikhil Khushalani, MD, discusses the benefits and limitations of fixed-dose treatment regimens in patients with unresectable or metastatic melanoma.

The FDA has received a biologics license application resubmission for cosibelimab in locally advanced or metastatic cutaneous squamous cell carcinoma.

Axel Hauschild, MD, PhD, discusses the role of adjuvant and neoadjuvant therapy in melanoma, highlighting the utility of perioperative approaches.

Ahmad Tarhini, MD, PhD, discusses a prognostic model evaluating patients with advanced melanoma treated with immune checkpoint inhibitors.

A 3 year survival update on the RELATIVITY-047 trial (NCT03470922), a global, randomized, double-blind phase 2/3 study comparing nivolumab plus relatlimab vs nivolumab in previously untreated metastatic or unresectable stage III or IV melanoma.

COMBI-AD (NCT01682083) evaluated dabrafenib plus trametinib for patients with BRAF-mutated AJCC-stage III melanoma, and here the final results for RFS, distant-metastasis-free survival and overall survival are presented.

PIVOTAL (NCT02938299) is an open label, randomized, multicenter, phase 3 trial evaluating Daromun as a neoadjuvant intralesional therapy for resectable, locally advanced Stage III melanoma.

UV1 added to pembrolizumab elicited sustained OS outcomes as frontline therapy in advanced, unresectable, or metastatic malignant melanoma.

The combination of RP1 and nivolumab was safe and effective in patients with anti–PD-1-failed advanced melanoma.

Neoadjuvant nivolumab/ipilimumab followed by TLND and response-driven adjuvant therapy reduced the risk of progression, recurrence, or death in melanoma.

Induction encorafenib/binimetinib before nivolumab/ipilimumab did not improve PFS in unresectable or metastatic BRAF V600E/K–positive melanoma.

The COMBI-AD dataset is the longest follow-up to date of adjuvant treatment for patients with stage III melanoma.