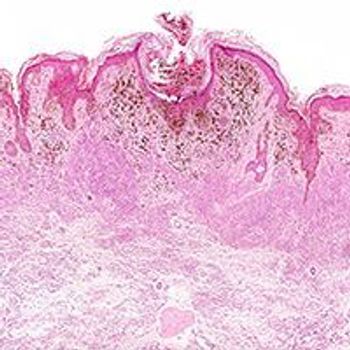
Sunitinib demonstrated synergistic activity with the BET inhibitors JQ1 and NHWD-870 in melanoma cell lines.

Your AI-Trained Oncology Knowledge Connection!


Associate Editor, OncLive
Ashling Wahner joined MJH Life Sciences in 2022. She produces OncLive's podcast, OncLive On Air, and helps ensure timely publication of news content and announcements from the FDA approval pipeline. She also attends conferences live and virtually to conduct video interviews and produce written coverage. Email: awahner@mjhlifesciences.com

Sunitinib demonstrated synergistic activity with the BET inhibitors JQ1 and NHWD-870 in melanoma cell lines.

Kevin Kalinsky, MD, MS, discusses genetic sequencing in triple-negative breast cancer, the significance of breast cancer tumor board discussions for informing further research and optimizing patient care, and how future directions in breast cancer treatment are building off the current benefits of drugs classes like antibody-drug conjugates.

Eunice Wang, MD, discusses the current standards for classifying and treating unfit patients with AML, treatment considerations for patients unfit for hypomethylating agents, and the potential role of investigational menin inhibitors and immunotherapies in this population.

RBN-2397, a PARP7 inhibitor, was found to be well tolerated and have preliminary antitumor activity as monotherapy in patients with advanced solid tumors, including head and neck squamous cell carcinoma, breast cancer, and squamous cell carcinoma of the lung.

The combination of nivolumab and relatlimab demonstrated clinical benefit with a manageable safety profile in patients with advanced melanoma who have progressed on prior PD-L1 or PD-1 inhibitors, regardless of PD-L1 and LAG-3 expression, according to findings from the ongoing phase 1/2a RELATIVITY-020 trial.

Erin K. Crane, MD, MPH, describes practice-changing findings from upfront trials with niraparib and olaparib, contextualizes the significance of the manufacturer restrictions of later-line PARP inhibitors for ovarian cancer, and predicts further research efforts in this population.

Daryl Pritchard, PhD, discussed how recent findings highlight the importance of targeted therapy in advanced lung cancer, areas where the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology could be updated to better encapsulate real-world practice patterns, and the ways in which increased access to molecular testing can help usher in a new era of personalized medicine.

Heterogeneous patterns of polycythemia vera management indicate the need for continued research in this disease and increased guideline specificity across treatment centers in Italy.

Jubilee Brown, MD, discusses future considerations for using minimally invasive surgery and PARP inhibitors in ovarian cancer, how immunotherapies have fulfilled an unmet need in cervical cancer, and the importance of leveraging molecular profiling to determine personalized therapeutic strategies in endometrial cancer.

Treatment with ruxolitinib impaired antibody responses to complete vaccination with the BNT162b2 SARS-CoV-2 vaccine in patients with myelofibrosis or polycythemia vera.

Susan M. Domchek, MD, highlights key points related to her presentation on germline testing and breast cancer treatment.

Treatment with ruxolitinib significantly reduced hematocrit levels and the number of yearly phlebotomies in patients with polycythemia vera, according to findings from a prespecified futility analysis of the phase 2b RuxoBEAT trial.

The combination of savolitinib and durvalumab induced high response rates with a manageable safety profile in patients with metastatic papillary renal cancer with MET-driven disease enrolled to the phase 2 CALYPSO trial, although the trial missed its primary end point of confirmed response rate for the overall study population.

Chandler Park, MD, MSc, FACP, discusses the evolution of metastatic hormone-sensitive prostate cancer treatment, shifting definitions of metastatic and nonmetastatic castration-resistant prostate cancer, the importance of antibody-drug conjugates in bladder cancer, and the future of biomarkers in renal cell carcinoma.

Brian I. Rini, MD, discusses standard and investigational treatment options for patients with RCC who are refractory to immunotherapy; the potential role of biomarkers in future targeted treatments; and the need for novel mechanisms such as HIF inhibition to meet the needs of patients who don’t respond to immunotherapy or VEGF TKIs.

James J. Harding, MD, describes the GI tumors where TSC1 or TSC2 alterations can arise, how the mechanism of action of nab-sirolimus may help fulfill an unmet need in this population, and the importance of using genetic testing to identify patients with mutations in TSC1, TSC2, or other genes to drive targeted treatment.

Tian Zhang, MD, MHS, discusses findings with single-agent PARP inhibitors within homologous recombination repair–deficient metastatic castration-resistant prostate cancer and describes how these advances pave the way for combining PARP inhibitors with hormonal therapies.

Michael S. Cookson, MD, MMHC, explains how discrepancies in defining biochemical recurrence are sparking a conversation about creating more consistent definitions that could allow for more widely applicable standards of care in prostate cancer .
Second infusion with tisagenlecleucel after prior tisagenlecleucel infusion produced short durations of minimal residual disease-negative responses in children and young adults with B-cell acute lymphoblastic leukemia.

First-line atezolizumab plus platinum-based chemotherapy and gemcitabine trended toward improved overall survival compared with placebo plus chemotherapy in patients with locally advanced or metastatic urothelial carcinoma.
Checkpoint inhibitor–based salvage regimens significantly reduced the likelihood that patients with relapsed/refractory classic Hodgkin lymphoma undergoing transplant would need further salvage therapy vs conventional salvage regimens.

Treatment with neoadjuvant nivolumab led to encouraging 5-year recurrence-free survival and overall survival rates compared with historical outcomes in patients with non–small cell lung cancer who underwent surgical resection.

Treatment with brexucabtagene autoleucel in the standard-of-care relapsed/refractory mantle cell lymphoma setting provided an efficacy and safety profile consistent with data reported in the phase 2 ZUMA-2 trial.

Rohit Kumar, MD, discusses the benefits of TKI/immunotherapy doublets in renal cell carcinoma, unmet needs regarding future research with triplet therapies in the frontline setting, and how hypoxia inducible factor inhibitors may play a role in the treatment of patients who have progressed on prior TKI/immunotherapy combinations.
The combination of camrelizumab and apatinib led to long-term antitumor activity with manageable toxicities in patients with recurrent or metastatic nasopharyngeal carcinoma.

Emma L. Barber, MD, emphasized the importance of the ongoing phase 3 PORTEC-4a trial, which is investigating the role of molecular risk profiles in determining whether patients with endometrial cancer should receive no adjuvant therapy, vaginal brachytherapy, or external beam radiotherapy.

Bartosz Chmielowski, MD, discusses the rationale for IGNYTE, the unique mechanism of action of RP1, and how melanoma research is seeking to determine treatments for patients based on their individual resistance mechanisms.

The selective small molecule MDM2 inhibitor milademetan administered at an intermittent dosing schedule was tolerable and showed early efficacy in patients with advanced cancers, according to findings from a phase 1 study.

Natalie S. Callander, MD, contextualizes the implications of the phase 3 ATLAS trial findings alongside other trials investigating maintenance regimens with triplet therapies in multiple myeloma.

Radiation plus docetaxel improved disease-free survival and overall survival vs radiation alone in patients with head and neck squamous cell carcinoma unsuitable for cisplatin-based chemoradiation, regardless of patients’ prespecified prognostic groups, according to findings from a phase 2/3 trial.