Precision Medicine in Oncology®
Latest News
Latest Videos

CME Content
More News









Ramona Dadu, MD, assistant professor, Department of Endocrine Neoplasia and Hormonal Disorders, Division of Internal Medicine, The University of Texas MD Anderson Cancer Center, discusses treatment approaches in medullary thyroid cancer.

The FDA has granted a priority review to a new drug application for the PARP 1/2 inhibitor niraparib for use as a maintenance therapy in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who have responded to platinum-based chemotherapy.

The FDA has approved rucaparib (Rubraca) as a treatment for patients with BRCA-positive advanced ovarian cancer who have received at least 2 prior lines of chemotherapy, according to Clovis, the manufacturer of the PARP inhibitor
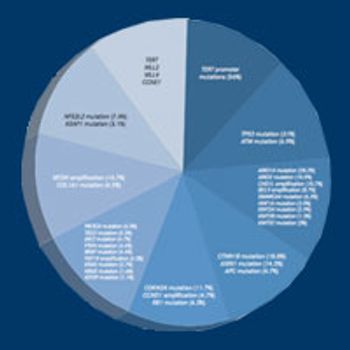
The growing incidence of primary liver cancer in the United States poses a great therapeutic challenge.

To great fanfare, Hackensack Meridian Health of New Jersey and Memorial Sloan Kettering Cancer Center announced a co-branding partnership that they say would lead to highly fruitful collaborative research and make hundreds of clinical trial opportunities available to their patients.

President Barack Obama signed the 21st Century Cures Act into law today, earmarking $6.3 billion over 10 years for advancements in precision medicine development, brain research, heroin and prescription drug abuse prevention, and mental health.

Véronique Diéras, MD, Department of Clinical Research, Institut Curie Paris & Saint Cloud, discusses results of a phase II trial exploring the combination of the PARP inhibitor veliparib with chemotherapy in patients with BRCA1/2-mutant breast cancer.