CAR T-cell Therapy
Latest News
Latest Videos

CME Content
More News

CMS has proposed a different way to handle payment for chimeric antigen receptor T-cell therapy, one that the medical and manufacturing community thinks is unworkable.

The CD19-targeted CAR T-cell product axicabtagene ciloleucel (axi-cel; Yescarta) is currently being investigated in patients with relapsed/refractory MCL in the ongoing ZUMA-2 trial.

Several studies presented at the 2018 ASCO Annual Meeting helped further refine and inform treatment strategies for the budding class of CAR T-cell therapies, with a focus on predicting adverse events and optimizing efficacy.

bb2121 had a median progression-free survival of 11.8 months and a median duration of response of 10.8 months for patients with relapsed/refractory heavily pretreated multiple myeloma.

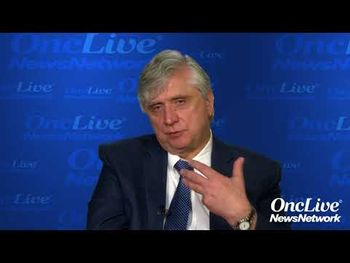

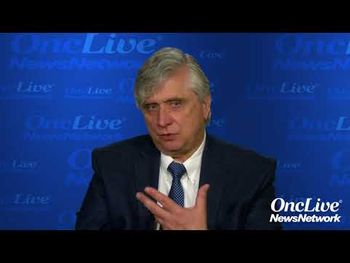






Alexander E. Perl, MD, associate professor of medicine, University of Pennsylvania, discusses remaining challenges with chimeric antigen receptor (CAR) T-cell therapy.

Anas Younes, MD, discusses the current and future state of CAR T-cell therapies and immune checkpoint inhibitors in the landscape of hematologic malignancies.

CMS's initial payment codes for CAR T-cell therapies are opposed by the medical community on the basis that they would be cumbersome to implement and wouldn't reflect the full amount of care delivered to each patient.

Renier J. Brentjens, MD, PhD, associate professor, chief, Cellular Therapeutics Center, Memorial Sloan Kettering Cancer Center, discusses targets for chimeric antigen receptor (CAR) T-cell therapy.

A first-time study of chimeric antigen receptor T-cell therapy in youths with Down syndrome-associated relapsed/refractory (r/r) acute lymphoblastic leukemia produced high remission rates and toxicity results that were similar to those observed in patients with r/r ALL.

Eliminating T cells carrying alpha and beta chains of the T-cell receptor reduced the risk for graft-versus-host-disease while producing "excellent" engraftment kinetics for young patients with acute leukemia who underwent hematopoietic stem cell transplant.

Results from a recent study may show why some patients with chronic lymphocytic leukemia are resistant to tisagenlecleucel, while potentially offering a pathway to enhance patient response.
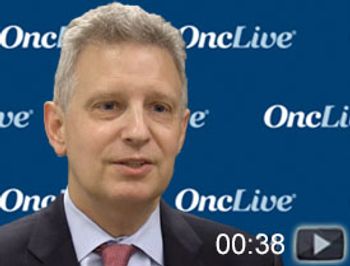
Ian W. Flinn, MD, PhD, director of Lymphoma Research, principal investigator, Sarah Cannon Research Institute, discusses the FDA approval of the chimeric antigen receptor T-cell therapy tisagenlecleucel (Kymriah) for use in adult patients with relapsed/refractory large B-cell lymphoma—including diffuse large B-cell lymphoma.

The FDA has approved tisagenlecleucel (Kymriah) for use in adult patients with relapsed/refractory large B-cell lymphoma—including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma—after 2 or more lines of systemic therapy.

Maung Myo Htut, MD, discusses the use of CAR T-cell therapy in multiple myeloma and the potential infusion of immunotherapy into treatment.