Ovarian Cancer
Latest News
Latest Videos

CME Content
More News

A retrospective analysis of the phase III GOG-0218 trial has identified a potential biomarker of response for bevacizumab in patients with advanced ovarian cancer.

Treatment with the PARP inhibitor rucaparib demonstrated robust clinical activity and a tolerable safety profile for women with biomarker-defined relapsed ovarian cancer

Monoclonal antibodies against PD-1 and PD-L1 have demonstrated encouraging signs of antitumor activity for patients with pretreated advanced ovarian cancer.

Robert L. Coleman, MD, FACOG, FACS, professor, Department of Gynecologic Oncology and Reproductive Medicine, University of Texas MD Anderson Cancer Center, discusses how bevacizumab could be effective in tumor types beyond ovarian cancer.

Optimal testing strategies for breast and ovarian cancer associated with mutations in the BRCA1/2 genes, including when to start testing and which prophylactic approach to pursue, have remained a topic of debate.
Joanne Xiu, PhD, research scientist, Caris Life Sciences, discusses findings from a study that showed hormone receptor (HR) expression is present in a subset of patients with uterine carcinosarcoma.

In an interview with OncLive, Robert C. Bast, Jr, MD, discussed his research using CA-125 levels and p53-autoantibodies as biomarkers for early detection of ovarian cancer.

Robert C. Bast, Jr., MD, vice president, Translational Research, The University of Texas MD Anderson Cancer Center, discusses a study that could help in early detection of ovarian cancer before CA-125 rises in patients.

The combination of olaparib with the novel AKT-targeting agent AZD5363 generated responses in a variety of tumor types among patients with and without BRCA1/2 mutations, demonstrating that a simultaneous attack on the two pathways is a safe and potentially versatile strategy.

A two-pronged strategy combining the PARP inhibitor olaparib and the PI3K inhibitor BKM120 proved to be a safe and clinically beneficial regimen for women with triple-negative breast cancer and for patients with high-grade serous ovarian cancer.

Clinical trial evidence indicates that some women with high-grade serous ovarian cancer who do not test positive for a BRCA mutation also may respond to olaparib.

Maurie Markman, MD, national director, Cancer Treatment Centers of America, discusses what he sees as future treatment paradigms in ovarian cancer.
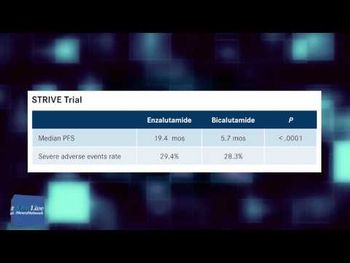

Ursula A. Matulonis, MD, medical director, gynecologic oncology, Susan F. Smith Center for Women's Cancers, institute physician, Dana-Farber Cancer Institute, discusses managing toxicities associated with olaparib for patients with ovarian cancer.

The FDA has granted a breakthrough therapy designation to rucaparib as a treatment for women with BRCA-mutated advanced ovarian cancer who have received at least two prior lines of platinum-based chemotherapy.

Fosbretabulin plus bevacizumab lowered the risk of progression by 31.5% but doubled the rate of hypertension compared with bevacizumab alone in patients with recurrent ovarian cancer.

Treatment with bevacizumab plus a chemotherapy doublet extended overall survival by nearly 5 months compared with chemotherapy alone for women with platinum-sensitive recurrent ovarian cancer.

Treatment with the immunotherapy Vigil delayed time to progression in all patients with stage III/IV ovarian cancer who were treated with the autologous tumor cell vaccine compared with those who were not in an open-label phase II trial.

Analyses of clinical trials continue to illuminate the role of the PARP inhibitor olaparib in the treatment of women with ovarian cancer, according to Ursula A. Matulonis, MD.

BJ Rimel, MD, discuss the current literature surrounding antiangiogenesis agents in ovarian cancer.

In an interview with OncLive, Don Dizon, MD, explains the advantages of IP therapy and why further research and education are necessary to bring it to the forefront of ovarian cancer treatment.

Recent advancements in the treatment of ovarian cancer, including surgical techniques, the approvals of bevacizumab and olaparib, and intraperitoneal chemotherapy, have led the National Comprehensive Cancer Network to make changes to their clinical practice guidelines.

Inhibiting EZH2 may improve outcomes in patients with clear cell ovarian carcinoma who harbor a ARID1A mutation.

OncLive spoke with Sanaz Memarzadeh, MD, on why ovarian and endometrial cancers are so challenging to treat, and what future research is needed to better understand these diseases.

The risk of developing the two most common types of ovarian cancer significantly increases when taking hormone replacement therapy to alleviate symptoms of menopause, according to a meta-analysis of epidemiological studies.