Ovarian Cancer
Latest News

Latest Videos

CME Content
More News

For the first time, physicians have a clearer understanding of the optimum age for prophylactic oophorectomy in patients with BRCA mutations who want to reduce their risk of ovarian, fallopian tube, and breast cancer.

Bradley Monk, MD, gynecologic oncologist, University of Arizona Cancer Center Phoenix Branch, on clinical trial endpoints and the approval of agents for ovarian cancer.

The renaissance in immunology is already affecting treatment paradigms for a variety of gynecologic cancers, and the impact is only likely to expand.

The FDA's rules for approving new drugs for the treatment of women with ovarian cancer pose frustrating problems for researchers developing novel agents and for clinicians seeking to improve outcomes for their patients

Franco M. Muggia, MD, professor of oncology, New York University, director, Division of Medical Oncology, New York University Medical Center, discusses using Doxil to treat ovarian cancer.

Jerry Lanchbury, PhD, Chief Scientific Officer, Myriad Genetics, Inc., describes the HRD (homologous recombination deficiency) test.

Antiangiogenic agents hold promise in gynecologic cancers, as evidenced by their single-agent activity in malignancies including ovarian cancer, recurrent endometrial cancer, and cervical cancer.

The genomic instability inherent in serous ovarian cancer poses treatment challenges, but it also represents a target which can be exploited through the use of PARP inhibitors
Bradley J. Monk, MD, gynecologic oncologist, University of Arizona Cancer Center Phoenix Branch, discusses the TRINOVA-1 trial.

Franco M. Muggia, MD, professor of oncology, New York University, director, Division of Medical Oncology, New York University Medical Center, discusses the challenge of developing targeted therapies to treat ovarian cancer.
Mark A. Morgan, MD, professor of obstetrics and gynecology, University of Pennsylvania, director, Gynecology Oncology for University of Pennsylvania Health Systems, discusses bulk reduction surgery prior to chemotherapy in patients with stage III ovarian cancer.

Robert A. Burger, MD, Professor, Department of Surgical Oncology, Director, Women's Cancer Center, Fox Chase Cancer Center, discusses the toxicity profile of bevacizumab when used to treat patients with ovarian cancer.

James "Tate" T. Thigpen, MD, professor of medicine, director of medical oncology, University of Mississippi School of Medicine, discusses the use of agents in ovarian cancer that are not approved by the FDA.
Michael Birrer, MD, PhD, professor of medicine, Harvard Medical School, director, Gillette Center for Gynecologic Oncology, Massachusetts General Hospital, discusses the role of PARP inhibitors in ovarian cancer.

Russell J. Schilder, MD, director, Gynecologic Medical Oncology, Thomas Jefferson University, Kimmel Cancer Center, discusses PARP inhibitors and their potential role in gynecologic cancers.
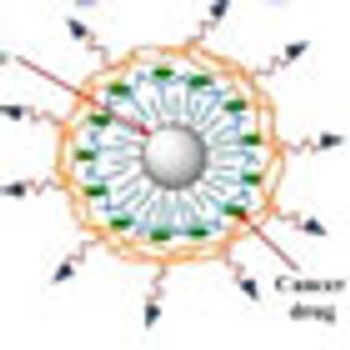
The combination of heat, chemotherapeutic drugs and an innovative delivery system based on nanotechnology may significantly improve the treatment of ovarian cancer while reducing side effects from toxic drugs.
Michael Birrer, MD, PhD, professor of medicine, Harvard Medical School, director, Gillette Center for Gynecologic Oncology, Massachusetts General Hospital, explains how the endpoints of progression-free survival (PFS) and overall survival (OS) are holding back the approval of drugs for patients with ovarian cancer.
Mark A. Morgan, MD, professor of obstetrics and gynecology, University of Pennsylvania, director, Gynecology Oncology for University of Pennsylvania Health Systems, discusses the role of robotic surgery in gynecologic cancer.

Access to Doxil has been inconsistent since mid-2011, when the drug's sole manufacturer announced a voluntary shutdown of production to address significant manufacturing and quality concerns.

Robert A. Burger, MD, FACOG, FACS, Professor, Department of Surgical Oncology, Director, Women's Cancer Center, Fox Chase Cancer Center, discusses the use of bevacizumab in patients with ovarian cancer.
Cancer stem cells are an underlying cause of a tumor's ability to recur and metastasize even after initial treatment. Therefore, targeting those cancer stem cells could prove to be a valuable tool in the treatment of several different tumor types.

James "Tate" T. Thigpen, MD, Professor of Medicine, Director, Division of Medical Oncology, University of Mississippi School of Medicine, discusses the comparison of treatment options in ovarian cancer.

Trebananib added to paclitaxel significantly improved progression-free survival in patients with recurrent ovarian cancer compared with placebo plus paclitaxel in the large international TRINOVA-1 trial.

Maurie Markman, MD, from the Cancer Treatment Centers of America, discusses the discovery of mutations and targets in ovarian cancer and the current progress being made in the development of a "breakthrough" agent for ovarian cancer.

Hot topics, emerging therapies, and tough cases from the front lines of patient care will be featured during the 9th Annual International Symposium on Ovarian Cancer and Gynecologic Malignancies, scheduled for October 5 in Philadelphia.