
Patients with non–small cell lung cancer and baseline liver metastases had improved progression-free survival and overall survival with the addition of immunotherapy to bevacizumab and a chemotherapy doublet.

Your AI-Trained Oncology Knowledge Connection!


Patients with non–small cell lung cancer and baseline liver metastases had improved progression-free survival and overall survival with the addition of immunotherapy to bevacizumab and a chemotherapy doublet.

Two highly selective MET inhibitors, tepotinib and capmatinib showed promising clinical activity in the first- and second-line treatment of patients with MET exon 14-altered advanced non–small cell lung cancer.

Adding ramucirumab to erlotinib reduced the risk of disease progression or death by over 40% versus erlotinib alone as a frontline treatment for patients with EGFR-positive NSCLC.

The FDA has unveiled what it describes as a pilot “concierge service” for oncologists and patients seeking information and assistance with expanded access to investigational therapies.

Neoadjuvant immunotherapy demonstrated substantial activity with a favorable toxicity profile in findings from 2 studies of patients with operable early-stage non–small cell lung cancer reported at the 2019 ASCO Annual Meeting.

More than 70% of female gynecologic oncologists in the United States and more than half of male practitioners have experienced sexual harassment in training or practice, according to results from a survey conducted by the Society of Gynecologic Oncology.

Similar long-term rates of survival were seen in patients with colorectal cancer and liver metastases regardless of whether the patient underwent laparoscopic or open liver surgery.

A broader set of clinical trial eligibility criteria proposed by the American Society of Clinical Oncology and Friends of Cancer Research would nearly double the number of patients with advanced non-small cell lung cancer available for enrollment.

Enfortumab vedotin induced responses in 44% of patients with locally advanced or metastatic urothelial cancer, including 12% experiencing a complete response to treatment.
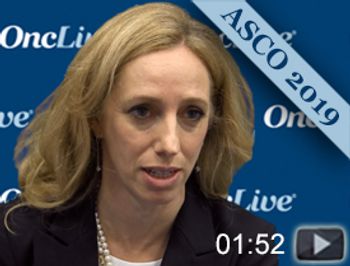
Talia Golan, MD, head of Sheba Pancreatic Cancer Center, discusses the results of the phase III POLO trial, which evaluated maintenance olaparib compared with placebo among patients with germline BRCA-mutated metastatic pancreatic cancer.
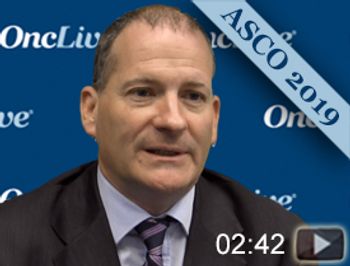
Christopher Sweeney, MBBS, medical oncologist at the Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute, discusses results of the phase III ENZAMET trial, which looked at standard therapy with or without enzalutamide as a treatment for patients with metastatic hormone-sensitive prostate cancer.

Adding isatuximab to pomalidomide and low-dose dexamethasone led to a greater than 40% reduction in the risk of disease progression or death compared with pomalidomide and dexamethasone alone in patients with relapsed/refractory multiple myeloma.

A subcutaneous flat-dose version of daratumumab demonstrated noninferior efficacy with a reduction in the treatment burden compared with the original intravenous formulation of the anti-CD38 monoclonal antibody for patients with relapsed/refractory multiple myeloma.

Examination of a cohort of patients with metastatic breast cancer and high mutational burden who were enrolled in the phase II Targeted Agent and Profiling Utilization Registry basket study shows that pembrolizumab monotherapy has antitumor activity in this population of heavily pretreated patients.

Lurbinectedin monotherapy achieved an overall response rate of 35.2% as a second-line treatment for patients with small cell lung cancer, according to findings from a phase II basket trial presented at the 2019 ASCO Annual Meeting.

Maintenance therapy with olaparib significantly improved progression-free survival compared with placebo among patients with germline BRCA-mutated metastatic pancreatic cancer.

Almost 80% of men with metastatic hormone-sensitive prostate cancer treated with enzalutamide plus standard of care (SOC) were alive after 3 years compared with 72% of patients who received a different non-steroidal anti-androgen plus SOC.

Higher income was associated with significantly greater probability of survival for patients with multiple myeloma, according to authors of a study they performed to call attention to the need to address socioeconomic disparities that influence treatment outcomes.

The expansion of Medicaid nearly eliminated racial disparities in time to cancer treatment.

Pre-Medicare-aged women with ovarian cancer were more likely to be diagnosed at an early stage and receive treatment within 30 days of diagnosis after passage of the Affordable Care Act than before.
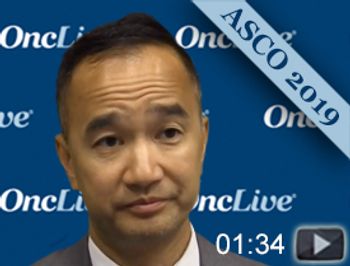
Kim N. Chi, MD, associate director of clinical research, Vancouver Prostate Centre, professor, Department of Medicine, University of British Columbia, discusses the results of the phase III TITAN trial, which evaluated apalutamide versus placebo (PBO) in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) receiving androgen deprivation therapy (ADT).
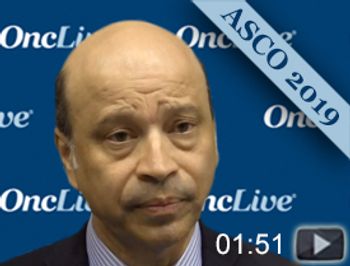
Debu Tripathy, MD, professor and chairman, Department of Breast Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, discusses the MONALEESA-7 trial in hormone receptor-positive breast cancer.

A consortium of cancer care institutions released details on a prototype system designed to link electronic health record systems to provide a source of real-world patient information to guide research and improve cancer treatment.

The development of tyrosine kinase inhibitors has revolutionized the treatment of EGFR-mutant non–small cell lung cancer in recent years, but new strategies are needed to overcome resistance mechanisms that promote disease recurrence.

Adding apalutamide to androgen deprivation therapy (ADT) reduced the risk of the death by 33% in patients with metastatic castration-sensitive prostate cancer compared with ADT alone.

Frontline pembrolizumab demonstrated non-inferior overall survival compared with standard chemotherapy among patients with PD-L1–positive, HER2-negative, advanced gastric or gastroesophageal junction cancer.

Ribociclib plus endocrine therapy demonstrated an estimated overall survival rate of 70.2% at 42 months compared 46.0% for placebo and endocrine therapy in premenopausal women with HR-positive, HER2-negative advanced breast cancer.

After 5 years, newly diagnosed patients with advanced non–small cell lung cancer demonstrated an overall survival (OS) rate of 23.2% from treatment with pembrolizumab monotherapy and previously treated patients had an OS rate of 15.5%.

Andre Goy, MD, MS, chief, Division of Lymphoma, chairman and director, John Theurer Cancer Center, discusses updates in CAR T-cell therapy that are being presented at the 2019 ASCO Annual Meeting.

Neeraj Agarwal, MD, associate professor, Division of Oncology, Department of Medicine, University of Utah School of Medicine; and director, Genitourinary Oncology Program, Oncology Division, co-leader, Urologic Oncology Multidisciplinary Program, associate director of Clinical Trials, Huntsman Cancer Institute, discusses part 1 findings from the phase III TALAPRO-2 trial in metastatic castration-resistant prostate cancer.