
The top 5 OncLive TV videos of the week cover insights in myeloma, AML, Ph-positive ALL, pediatric low-grade glioma, and nonadvanced systemic mastocytosis.

Your AI-Trained Oncology Knowledge Connection!


The top 5 OncLive TV videos of the week cover insights in myeloma, AML, Ph-positive ALL, pediatric low-grade glioma, and nonadvanced systemic mastocytosis.
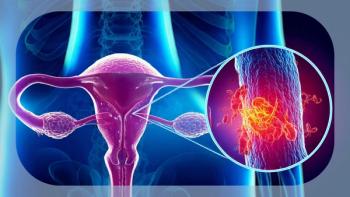
Ten-year data from the PORTEC-3 trial show that adjuvant chemoradiotherapy improves OS and RFS vs radiotherapy alone in high-risk endometrial cancer.

The FDA approved T-DXd plus pertuzumab in HER2+ breast cancer, subcutaneous amivantamab in EGFR+ NSCLC, and rucaparib in BRCA-mutated mCRPC.
Only 13% of US patients with CP-CML received FDA-recommended ponatinib dosing, highlighting gaps in dose optimization and the need for education.

Experts reflect on pivotal data, emerging agents, and highly-anticipated trends spanning CML, CLL, B-cell lymphomas, and other hematologic malignancies.
Experts convened during an OncLive Scientific Interchange and Workshop to discuss the evolving treatment landscapes in CSCC and melanoma.
The NMPA has accepted a new drug application for tinengotinib tablets for the treatment of patients with pretreated advanced/metastatic cholangiocarcinoma.

A new study presented at the 2025 ASH Annual Meeting revealed that subtle disruptions in genome architecture can predispose individuals to lymphoma.
Tumor burden and disease status were not correlated with CRS risk or severity in newly diagnosed, relapsed/refractory myeloma managed with elranatamab.

Adam Fox, MD, discusses the need for molecular profiling in non–small cell lung cancer and how these results factor into multidisciplinary planning.
Epcoritamab-based treatment yielded durable remissions in patients with follicular lymphoma, both as first-line induction and maintenance.

Phase 1 data showed that LP-184 displayed an acceptable safety profile and was well-tolerated in heavily-pretreated patients with advanced solid tumors.
Frontline dasatinib was linked to frequent T315I mutations at relapse in Ph+ ALL, while ponatinib showed fewer resistance mutations and favorable outcomes.

Breast oncology experts discuss SABCS 2025 data from HER2CLIMB-05 and lidERA that may influence maintenance therapy and adjuvant endocrine care.

Tafasitamab plus lenalidomide and rituximab has been approved by the European Commission in relapsed/refractory follicular lymphoma.
Signatera MRD positivity predicted improved DFS and OS with adjuvant celecoxib plus chemotherapy vs placebo in resected colorectal cancer.

Patient who are adolescent or young adults are at higher risk of metastatic recurrence according to researchers at UC Davis Health.
Safety analysis shows sacituzumab govitecan plus pembrolizumab has a manageable profile and fewer dose reductions than chemoimmunotherapy in PD-L1+ TNBC.
Epcoritamab plus R-CHOP delivered durable remissions beyond 3 years across high-risk subgroups of patients with newly diagnosed DLBCL.

The subcutaneous formulation of amivantamab has received FDA approval for refractory, EGFR-mutant non–small cell lung cancer.

The FDA has granted regular approval to rucaparib (Rubraca) for BRCA mutation–associated metastatic castration-resistant prostate cancer.

Alexander B. Olawaiye, MD, spotlights this phase 2 study evaluating bevacizumab plus relacorilant and nab-paclitaxel in platinum-resistant ovarian cancer.

Eniluracil plus capecitabine showed signs of improved efficacy vs capecitabine alone while maintaining safety in advanced/metastatic breast cancer.

Enfortumab vedotin plus pembrolizumab is the first perioperative regimen without chemotherapy to improve both EFS and OS in cisplatin-eligible MIBC.

FOG-001 demonstrated a manageable safety profile in addition to antitumor activity that was clinically meaningful in patients with desmoid tumors.

IL-36 gamma ‘armored’ CAR T cells boost effectiveness of therapies and help eradicate solid tumors according to researchers at Roswell Park.

Maurie Markman, MD, discusses the role of decision support tools in cancer management.
Ciforadenant in combination with ipilimumab and nivolumab was safe and feasible but did not enhance activity in frontline ccRCC.

R. Lor Randall, MD, FACS, discusses findings from a phase 2 study of denosumab in patients with osteosarcoma.

The FDA has expanded the indications of 2 assays to identify patients with HER2+ breast cancer for treatment with T-DXd.