
Concurrent frontline therapy with cladribine and rituximab led to a significant improvement in minimal residual disease–free complete responses versus cladribine followed by delayed rituximab in patients with hairy cell leukemia.

Your AI-Trained Oncology Knowledge Connection!


Concurrent frontline therapy with cladribine and rituximab led to a significant improvement in minimal residual disease–free complete responses versus cladribine followed by delayed rituximab in patients with hairy cell leukemia.

Belumosudil (KD025) continued to show high levels of clinical activity in patients with previously treated chronic graft-versus-host disease.

Rana R. McKay, MD, sheds light on the impact of age and gender on the outcomes of patients with RCC, the implications of clinical determinants on toxicity, and where future research efforts will focus.

Alan H. Bryce, MD, discusses the recent regulatory approval of rucaparib in metastatic castration-resistant prostate cancer and explains how the approval has underscored the importance of genetic testing in the field.

Mario Sznol, MD, discusses the current immunotherapy paradigm in melanoma and promising agents that are in development.

Alan H. Bryce, MD, discusses the recent regulatory approval of rucaparib in metastatic castration-resistant prostate cancer and explains how the approval has underscored the importance of genetic testing in the field.

A supplemental New Drug Application has been submitted to the FDA for the use of selinexor as a treatment for patients with multiple myeloma following at least 1 line of prior therapy.

Neeraj Agarwal, MD, provides insight into olaparib, further discussed the data that led to the regulatory approval, and shared the implications of this approval in metastatic castration-resistant prostate cancer.

The FDA has approved two companion diagnostics to identify patients with metastatic castration-resistant prostate cancer with homologous recombination repair mutations, making them eligible for treatment with olaparib.

Brian M. Slomovitz, MD, explaines the need to determine optimal sequencing strategies in endometrial cancer.

Brian I. Rini, MD, speaks about the TIVO-3 trial and how its results could provide patients with another “weapon in this disease.”

The FDA has approved olaparib for the treatment of adult patients with deleterious or suspected deleterious germline or somatic homologous recombination repair gene–mutated metastatic castration-resistant prostate cancer who have progressed following prior treatment with enzalutamide or abiraterone acetate.
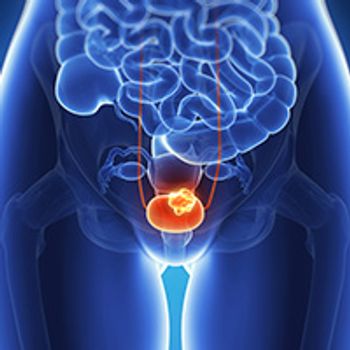
Low-intensity surveillance may be comparable to high-intensity surveillance for cancer control in patients with high-risk non-muscle invasive bladder cancer.

Pembrolizumab combined with trastuzumab and chemotherapy demonstrated promising clinical activity in patients with HER2-positive metastatic esophagogastric cancer.

Preetesh Jain, MD, PhD, evaluates mechanisms of resistance to venetoclax in patients with heavily pretreated mantle cell lymphoma and the next phase of this research.

Jamie E. Chaft, MD, provides insight into IRAEs and immunotherapy restart, considerations for immunotherapy in the pre- and post-operative period, outlines biomarker research in lung cancer, and projects her hopes for the future use of this approach in this space.

The FDA has approved PD-L1 IHC 28-8 pharmDx for use as a companion diagnostic to identify patients with metastatic non–small cell lung cancer with PD-L1 tumor expression ≥1%, making them eligible for frontline treatment with nivolumab plus ipilimumab.

The FDA has granted a breakthrough therapy designation to fam-trastuzumab deruxtecan-nxki for the treatment of patients with HER2-positive metastatic non–small cell lung cancer.

Mary-Beth Percival, MD, provides insight into precautionary measures put into place to ensure patient safety, some of the considerations included in the paper, and the different ways in which she is overcoming challenges faced in practice in light of the pandemic.

John O. Mascarenhas, MD, discusses patient characteristics and outcomes following ruxolitinib discontinuation in myelofibrosis and research efforts being made to address a large unmet need in the space.

Mary-Beth Percival, MD, provides insight into precautionary measures put into place to ensure patient safety, some of the considerations included in the paper, and the different ways in which she is overcoming challenges faced in practice in light of the pandemic.

Michael Wang, MD, discusses paradigm shifts in lymphomas and the research efforts driving precision medicine in mantle cell lymphoma.

Michael Wang, MD, discusses paradigm shifts in lymphomas and the research efforts driving precision medicine in mantle cell lymphoma.

Nicholas J. Vogelzang, MD, discusses the significance of the rucaparib approval for this patient population.

The FDA has approved ripretinib (Qinlock) for the fourth-line treatment of patients with advanced gastrointestinal stromal tumor.

The FDA has approved the combination of nivolumab and ipilimumab for the first-line treatment of patients with PD-L1–positive metastatic or recurrent non–small cell lung cancer that does not have EGFR or ALK genomic tumor aberrations.

The FDA has approved rucaparib for the treatment of adult patients with BRCA-mutant recurrent, metastatic castration-resistant prostate cancer.

Rushang D. Patel, MD, PhD, discusses the potential role of ruxolitinib in the treatment of patients with cGVHD.

The FDA has approved pomalidomide for patients with AIDS-related Kaposi sarcoma whose disease has become resistant to highly active antiretroviral therapy, or in patients with Kaposi sarcoma who are HIV-negative.

Ivosidenib induced a 63% reduction in the risk of disease progression or death versus placebo in previously treated patients with IDH1-mutant advanced cholangiocarcinoma, according to findings from the phase 3 ClarIDHy study published in the Lancet Oncology.